AFM1 in Milk: Physical, Biological, and Prophylactic Methods to Mitigate Contamination
Abstract
:1. Introduction
2. Carry-Over of AF in Milk
3. Toxicity of AFM1
4. Regulation and Monitoring
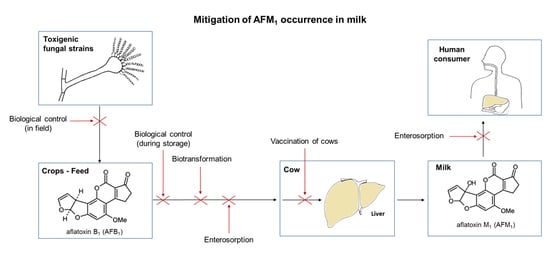
5. Mitigation of AFM1 Occurrence in Milk
5.1. Biological Control
5.1.1. Biocontrol in Field
5.1.2. Biocontrol during Storage of Feed
5.2. Enterosorption by Dietary Clay Minerals
5.2.1. Clay-Based Decontamination of Feed
5.2.2. Clay-Based Decontamination of Milk
5.3. Microbial Enterosorption
5.4. Biotransformation by Microorganisms or Enzymes
5.5. Neutralization by Specific Antibodies Induced by Vaccination
6. Conclusions
Author Contributions
Conflicts of Interest
References
- McLean, M.; Dutton, M.F. Cellular interactions and metabolism of aflatoxin: An update. Pharmacol. Ther. 1995, 65, 163–192. [Google Scholar] [CrossRef]
- Medina, A.; Rodriguez, A.; Magan, N. Effect of climate change on Aspergillus flavus and aflatoxin B1 production. Front. Microbiol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Sudakin, D.L. Dietary aflatoxin exposure and chemoprevention of cancer: A clinical review. J. Toxicol. Clin. Toxicol. 2003, 41, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.D.; Eaton, D.L.; Guengerich, F.P.; Coulombe, R.A., Jr. Aflatoxin B1 activation in human lung. Toxicol. Appl. Pharmacol. 1997, 144, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Jakab, G.J.; Hmieleski, R.R.; Zarba, A.; Hemenway, D.R.; Groopman, J.D. Respiratory aflatoxicosis: Suppression of pulmonary and systemic host defenses in rats and mice. Toxicol. Appl. Pharmacol. 1994, 125, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Dogra, R.K.; Khanna, S.K.; Das, M. Skin tumorigenic potential of aflatoxin B1 in mice. Food Chem. Toxicol. 2006, 44, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [PubMed]
- Guengerich, F.P.; Arneson, K.O.; Williams, K.M.; Deng, Z.; Harris, T.M. Reaction of aflatoxin B1 oxidation products with lysine. Chem. Res. Toxicol. 2002, 15, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Sabbioni, G.; Skipper, P.L.; Buchi, G.; Tannenbaum, S.R. Isolation and characterization of the major serum albumin adduct formed by aflatoxin B1 in vivo in rats. Carcinogenesis 1987, 8, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Neal, G.E.; Judah, D.J.; Stirpe, F.; Patterson, D.S. The formation of 2,3-dihydroxy-2,3-dihydro-aflatoxin B1 by the metabolism of aflatoxin B1 by liver microsomes isolated from certain avian and mammalian species and the possible role of this metabolite in the acute toxicity of aflatoxin B1. Toxicol. Appl. Pharmacol. 1981, 58, 431–437. [Google Scholar] [CrossRef]
- Shen, H.M.; Shi, C.Y.; Shen, Y.; Ong, C.N. Detection of elevated reactive oxygen species level in cultured rat hepatocytes treated with aflatoxin B1. Free Radic. Biol. Med. 1996, 21, 139–146. [Google Scholar] [CrossRef]
- Lewis, L.; Onsongo, M.; Njapau, H.; Schurz-Rogers, H.; Luber, G.; Kieszak, S.; Nyamongo, J.; Backer, L.; Dahiye, A.M.; Misore, A.; et al. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environ. Health Perspect. 2005, 113, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.J.; Wild, C.P. Epidemiology of aflatoxin-related disease. In The Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance; Eaton, D.L., Groopman, J.D., Eds.; Academic Press: San Diego, CA, USA, 1994; pp. 233–258. [Google Scholar]
- Azziz-Baumgartner, E.; Lindblade, K.; Gieseker, K.; Rogers, H.S.; Kieszak, S.; Njapau, H.; Schleicher, R.; McCoy, L.F.; Misore, A.; DeCock, K.; et al. Case-control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environ. Health Perspect. 2005, 113, 1779–1783. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; International Agency for Research on Cancer. Aflatoxins. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press: Lyon, France, 2002; Volume 82, pp. 171–300. [Google Scholar]
- Bressac, B.; Kew, M.; Wands, J.; Ozturk, M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature 1991, 350, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Strosnider, H.; Azziz-Baumgartner, E.; Banziger, M.; Bhat, R.V.; Breiman, R.; Brune, M.N.; DeCock, K.; Dilley, A.; Groopman, J.; Hell, K.; et al. Workgroup report: Public health strategies for reducing aflatoxin exposure in developing countries. Environ. Health Perspect. 2006, 114, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Robens, J.F.; Richard, J.L. Aflatoxins in animal and human health. Rev. Environ. Contam. Toxicol. 1992, 127, 69–94. [Google Scholar] [PubMed]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Völkel, I.; Schröer-Merker, E.; Czerny, C. The carry-over of mycotoxins in products of animal origin with special regard to its implications for the european food safety legislation. Food Nutr. Sci. 2011, 2, 852–867. [Google Scholar] [CrossRef]
- Roebuck, B.D.; Wogan, G.N. Species comparison of in vitro metabolism of aflatoxin B1. Cancer Res. 1977, 37, 1649–1656. [Google Scholar] [PubMed]
- Helferich, W.G.; Baldwin, R.L.; Hsieh, D.P. [14C]-aflatoxin B1 metabolism in lactating goats and rats. J. Anim. Sci. 1986, 62, 697–705. [Google Scholar] [PubMed]
- European Food Safety Agency Publication. Opinion of the Scientific Panel on contaminants in the food chain on a request from the Commission related to aflatoxin B1 as undesirable substance in animal feed: Request No EFSA-Q-2003–035. EFSA J. 2004, 39, 1–27. [Google Scholar]
- Diaz, D.E.; Hagler, W.M., Jr.; Blackwelder, J.T.; Eve, J.A.; Hopkins, B.A.; Anderson, K.L.; Jones, F.T.; Whitlow, L.W. Aflatoxin binders ii: Reduction of aflatoxin M1 in milk by sequestering agents of cows consuming aflatoxin in feed. Mycopathologia 2004, 157, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Battacone, G.; Nudda, A.; Cannas, A.; Borlino, A.C.; Bomboi, G.; Pulina, G. Excretion of aflatoxin M1 in milk of dairy ewes treated with different doses of aflatoxin B1. J. Dairy Sci. 2003, 86, 2667–2675. [Google Scholar] [CrossRef]
- Masoero, F.; Gallo, A.; Moschini, M.; Piva, G.; Diaz, D. Carryover of aflatoxin from feed to milk in dairy cows with low or high somatic cell counts. Animal 2007, 1, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Polan, C.E.; Hayes, J.R.; Campbell, T.C. Consumption and fate of aflatoxin B1 by lactating cows. J. Agric. Food Chem. 1974, 22, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Frobish, R.A.; Bradley, B.D.; Wagner, D.D.; Long-Bradley, P.E.; Hairston, H.J. Aflatoxin residues in milk of dairy cows after ingestion of naturally contaminated grain. J. Food Protect. 1986, 49, 781–785. [Google Scholar]
- Pettersson, H.; Bertilsson, J.; Wennberg, O. Carry-Over of Aflatoxin from Dairy Cattle Feed to Milk. Healthy Animals, Safe Foods, Healthy Man. In Proceedings of the World Association of Veterinary Food Hygienists: Xth Jubilee International Symposium, Stockholm, Sweden, 2–7 July 1989; pp. 97–102.
- Veldman, A.; Meijs, J.A.C.; Borggreve, G.J.; Heeres-van der Tol, J.J. Carry-over of aflatoxin from cows’ food to milk. Anim. Product. 1992, 55, 163–168. [Google Scholar] [CrossRef]
- Galvano, F.; Pietri, A.; Bertuzzi, T.; Fusconi, G.; Galvano, M.; Piva, A.; Piva, G. Reduction of carryover of aflatoxin from cow feed to milk by addition of activated carbons. J. Food Protect. 1996, 59, 551–554. [Google Scholar]
- Price, R.L.; Paulson, J.H.; Lough, O.G.; Ginng, C.; Kurtz, A.G. Aflatoxin conversion by dairy cattle consuming naturally-contaminated whole cottonseed. J. Food Protect. 1985, 48, 11–15. [Google Scholar]
- Munksgaard, L.; Larsen, J.; Werner, H.; Andersen, P.E.; Viuf, B.T. Carry over of aflatoxin from cows’ feed to milk and milk products. Milchwissenschaft 1987, 42, 165–167. [Google Scholar]
- Harvey, R.B.; Phillips, T.D.; Ellis, J.A.; Kubena, L.F.; Huff, W.E.; Petersen, H.D. Effects on aflatoxin M1 residues in milk by addition of hydrated sodium calcium aluminosilicate to aflatoxin-contaminated diets of dairy cows. Am. J. Vet. Res. 1991, 52, 1556–1559. [Google Scholar] [PubMed]
- Neal, G.E.; Eaton, D.L.; Judah, D.J.; Verma, A. Metabolism and toxicity of aflatoxins M1 and B1 in human-derived in vitro systems. Toxicol. Appl. Pharmacol. 1998, 151, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Ezekiel, C.N.; Sulyok, M.; Warth, B.; Odebode, A.C.; Krska, R. Natural occurrence of mycotoxins in peanut cake from Nigeria. Food Control 2012, 27, 338–342. [Google Scholar] [CrossRef]
- Streit, E.; Schwab, C.; Sulyok, M.; Naehrer, K.; Krska, R.; Schatzmayr, G. Multi-mycotoxin screening reveals the occurrence of 139 different secondary metabolites in feed and feed ingredients. Toxins 2013, 5, 504–523. [Google Scholar] [CrossRef] [PubMed]
- Matumba, L.; Sulyok, M.; Monjerezi, M.; Biswick, T.; Krska, R. Fungal metabolites diversity in maize and associated human dietary exposures relate to micro-climatic patterns in Malawi. World Mycotoxin J. 2015, 8, 269–282. [Google Scholar] [CrossRef]
- World Health Organization; International Agency for Research on Cancer. Aflatoxins. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press: Lyon, France, 1993; Volume 56, pp. 245–395. [Google Scholar]
- Allcroft, R.; Carnaghan, R.B.A. Groundnut toxicity: An examination for toxin in human food products from animals fed toxic groundnut meal. Vet. Rec. 1963, 75, 259–263. [Google Scholar]
- Pong, R.S.; Wogan, G.N. Toxicity and biochemical and fine effects of synthetic AFM1 and B1 in rat liver. J. Natl. Cancer Inst. 1971, 47, 585–590. [Google Scholar] [PubMed]
- Neal, G.E.; Colley, P.J. Some high-performance liquid-chromatographic studies of the metabolism of aflatoxins by rat liver microsomal preparations. Biochem. J. 1978, 174, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.J.; Hsieh, D.P. Mutagenicity of aflatoxins related to their metabolism and carcinogenic potential. Proc. Natl. Acad. Sci. USA 1976, 73, 2241–2244. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, T.; Ogawa, H.I.; Ryo, H.; Fujikawa, K. DNA-damaging potency and genotoxicity of aflatoxin M1 in somatic cells in vivo of Drosophila melanogaster. Mutagenesis 1995, 10, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, N.; Liu, J.; Li, F.D.; Li, S.L.; Wang, J.Q. Aflatoxin B1 and aflatoxin M1 induced cytotoxicity and DNA damage in differentiated and undifferentiated Caco-2 cells. Food Chem. Toxicol. 2015, 83, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Caloni, F.; Stammati, A.; Frigge, G.; de Angelis, I. Aflatoxin M1 absorption and cytotoxicity on human intestinal in vitro model. Toxicon 2006, 47, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Galvano, F.; Galofaro, V.; Galvano, G. Occurrence and stability of aflatoxin M1 in milk and milk products: A worldwide review. J. Food Protect. 1996, 59, 1079–1090. [Google Scholar]
- Bwibo, N.O.; Neumann, C.G. The need for animal source foods by kenyan children. J. Nutr. 2003, 133, 3936S–3940S. [Google Scholar] [PubMed]
- Food and Agriculture Organization of the United Nations. Worldwide Regulations for Mycotoxins in Food and Feed in 2003; Food and Nutrition Paper 81; FAO: Rome, Italy, 2004; pp. 17–23. [Google Scholar]
- Stoloff, L.; van Egmond, H.P.; Park, D.L. Rationales for the establishment of limits and regulations for mycotoxins. Food Addit. Contam. 1991, 8, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Wagacha, J.M.; Muthomi, J.W. Mycotoxin problem in Africa: Current status, implications to food safety and health and possible management strategies. Int. J. Food Microbiol. 2008, 124, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Center, M.M.; DeSantis, C.; Ward, E.M. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1893–1907. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Roebuck, B.D.; Wogan, G.N.; Groopman, J.D. Aflatoxin: A 50-year odyssey of mechanistic and translational toxicology. Toxicology 2011, 120, S28–S48. [Google Scholar] [CrossRef] [PubMed]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarc'h, A.; Lebrihi, A. Review of mycotoxin reduction in food and feed: From prevention in the field to detoxification by adsorption or transformation. Food Addit. Contam. Part A 2011, 28, 1590–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, K.R.N.; Reddy, C.S.; Muralidharan, K. Potential of botanicals and biocontrol agents on growth and aflatoxin production by Aspergillus flavus infecting rice grains. Food Control 2009, 20, 173–178. [Google Scholar] [CrossRef]
- Palumbo, J.D.; Baker, J.L.; Mahoney, N.E. Isolation of bacterial antagonists of Aspergillus flavus from almonds. Microb. Ecol. 2006, 52, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Nesci, A.V.; Bluma, R.V.; Etcheverry, M.G. In vitro selection of maize rhizobacteria to study potential biological control of Aspergillus section flavi and aflatoxin production. Eur. J. Plant Pathol. 2005, 113, 159–171. [Google Scholar] [CrossRef]
- Hua, S.S.; Baker, J.L.; Flores-Espiritu, M. Interactions of saprophytic yeasts with a nor mutant of Aspergillus flavus. Appl. Environ. Microbiol. 1999, 65, 2738–2740. [Google Scholar] [PubMed]
- Yin, Y.N.; Yan, L.Y.; Jiang, J.H.; Ma, Z.H. Biological control of aflatoxin contamination of crops. J. Zhejiang Univ. Sci. B 2008, 9, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Cotty, P.J.; Antilla, L.; Wakelyn, P.J. Competitive exclusion of aflatoxin producers: Farmer-driven research and development. In Biological Control: A Global Perspective; Vincent, C., Goettel, M.S., Lazarovits, G., Eds.; CAB International: Cambridge, MA, USA, 2007; pp. 241–253. [Google Scholar]
- Cotty, P.J.; Bhatnagar, D. Variability among atoxigenic Aspergillus flavus strains in ability to prevent aflatoxin contamination and production of aflatoxin biosynthetic pathway enzymes. Appl. Environ. Microbiol. 1994, 60, 2248–2251. [Google Scholar] [PubMed]
- Atehnkeng, J.; Ojiambo, P.S.; Ikotun, T.; Sikora, R.A.; Cotty, P.J.; Bandyopadhyay, R. Evaluation of atoxigenic isolates of Aspergillus flavus as potential biocontrol agents for aflatoxin in maize. Food Addit. Contam. Part A 2008, 25, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Hocking, A.D. Mycotoxins in Australia: Biocontrol of aflatoxin in peanuts. Mycopathologia 2006, 162, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Dorner, J.W.; Cole, R.J.; Wicklow, D.T. Aflatoxin reduction in corn through field application of competitive fungi. J. Food Prot. 1999, 62, 650–656. [Google Scholar] [PubMed]
- Dorner, J.W.; Lamb, M.C. Development and commercial use of Afla-Guard®, an aflatoxin biocontrol agent. Mycotoxin Res. 2006, 22, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, K.C. Non-aflatoxigenic Aspergillus flavus to prevent aflatoxin contamination in crops: Advantages and limitations. Front. Microbiol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, K.F.; Henry, S.H. Risk of exposure to and mitigation of effect of aflatoxin on human health: A west african example. J. Toxicol.: Toxin Rev. 2004, 23, 217–247. [Google Scholar] [CrossRef]
- Atehnkeng, J.; Ojiambo, P.S.; Donner, M.; Ikotun, T.; Sikora, R.A.; Cotty, P.J.; Bandyopadhyay, R. Distribution and toxigenicity of Aspergillus species isolated from maize kernels from three agro-ecological zones in Nigeria. Int. J. Food Microbiol. 2008, 122, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wei, D.-D.; Selvaraj, J.N.; Shang, B.; Zhang, C.-S.; Xing, F.-G.; Zhao, Y.-J.; Wang, Y.; Liu, Y. A strain of Aspergillus flavus from China shows potential as a biocontrol agent for aflatoxin contamination. Biocontrol Sci. Techn. 2014, 25, 583–592. [Google Scholar] [CrossRef]
- Ehrlich, K.C.; Moore, G.G.; Mellon, J.E.; Bhatnagar, D. Challenges facing the biological control strategy for eliminating aflatoxin contamination. World Mycotoxin J. 2015, 8, 225–233. [Google Scholar] [CrossRef]
- Alberts, J.F.; Gelderblom, W.C.; Botha, A.; van Zyl, W.H. Degradation of aflatoxin B1 by fungal laccase enzymes. Int. J. Food Microbiol. 2009, 135, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P.H.; Jespersen, L. Saccharomyces cerevisiae and lactic acid bacteria as potential mycotoxin decontaminating agents. Trends Food Sci. Technol. 2006, 17, 48–55. [Google Scholar] [CrossRef]
- Prado, G.; Madeira, J.E.; Morais, V.A.; Oliveira, M.S.; Souza, R.A.; Peluzio, J.M.; Godoy, I.J.; Silva, J.F.; Pimenta, R.S. Reduction of aflatoxin B1 in stored peanuts (Arachis hypogaea L.) using Saccharomyces cerevisiae. J. Food Prot. 2011, 74, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Armando, M.R.; Dogi, C.A.; Rosa, C.A.; Dalcero, A.M.; Cavaglieri, L.R. Saccharomyces cerevisiae strains and the reduction of Aspergillus parasiticus growth and aflatoxin B1 production at different interacting environmental conditions, in vitro. Food Addit. Contam. Part A 2012, 29, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Dalié, D.K.D.; Deschamps, A.M.; Richard-Forget, F. Lactic acid bacteria—Potential for control of mould growth and mycotoxins: A review. Food Control 2010, 21, 370–380. [Google Scholar] [CrossRef]
- Aryantha, N.P.; Lunggani, A.T. Suppression on the aflatoxin-B production and the growth of Aspergillus flavus by lactic acid bacteria (Lactobacillus delbrueckii, Lactobacillus fermentum and Lactobacillus plantarum). Biotechnology 2007, 6, 257–262. [Google Scholar]
- Gerbaldo, G.A.; Barberis, C.; Pascual, L.; Dalcero, A.; Barberis, L. Antifungal activity of two Lactobacillus strains with potential probiotic properties. FEMS Microbiol. Lett. 2012, 332, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Haskard, C.A.; El-Nezami, H.S.; Kankaanpää, P.E.; Salminen, S.; Ahokas, J.T. Surface binding of aflatoxin B1 by lactic acid bacteria. Appl. Environ. Microbiol. 2001, 67, 3086–3091. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, N.K.; Kung, L., Jr. The effect of Lactobacillus buchneri, Lactobacillus plantarum, or a chemical preservative on the fermentation and aerobic stability of corn silage. J. Dairy Sci. 2000, 83, 526–535. [Google Scholar] [CrossRef]
- Tabacco, E.; Piano, S.; Revello-Chion, A.; Borreani, G. Effect of Lactobacillus buchneri LN4637 and Lactobacillus buchneri LN40177 on the aerobic stability, fermentation products, and microbial populations of corn silage under farm conditions. J. Dairy Sci. 2011, 94, 5589–5598. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.F.M.; Peluzio, J.M.; Prado, G.; Madeira, J.E.; Silva, M.O.; de Morais, P.B.; Rosa, C.A.; Pimenta, R.S.; Nicoli, J.R. Use of probiotics to control aflatoxin production in peanut grains. Sci. World J. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kabak, B.; Dobson, A.D.; Var, I. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.D.; Clement, B.A.; Park, D.L. Approaches to Reduction of Aflatoxin in Foods and Feeds. In The Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance; Eaton, D.L., Groopman, J.D., Eds.; Academic Press: San Diego, CA, USA, 1994; pp. 383–406. [Google Scholar]
- Phillips, T.D.; Afriyie-Gyawu, E.; Williams, J.; Huebner, H.; Ankrah, N.A.; Ofori-Adjei, D.; Jolly, P.; Johnson, N.; Taylor, J.; Marroquin-Cardona, A.; et al. Reducing human exposure to aflatoxin through the use of clay: A review. Food Addit. Contam. Part A 2008, 25, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Kutz, R.E.; Sampson, J.D.; Pompeu, L.B.; Ledoux, D.R.; Spain, J.N.; Vazquez-Anon, M.; Rottinghaus, G.E. Efficacy of solis, novasilplus, and MTB-100 to reduce aflatoxin M1 levels in milk of early to mid lactation dairy cows fed aflatoxin B1. J. Dairy Sci. 2009, 92, 3959–3963. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, O.C.; Han, J.H.; Staples, C.R.; Adesogan, A.T. Effect of adding a mycotoxin-sequestering agent on milk aflatoxin M1 concentration and the performance and immune response of dairy cattle fed an aflatoxin B1-contaminated diet. J. Dairy Sci. 2012, 95, 5901–5908. [Google Scholar] [CrossRef] [PubMed]
- Kissell, L.; Davidson, S.; Hopkins, B.A.; Smith, G.W.; Whitlow, L.W. Effect of experimental feed additives on aflatoxin in milk of dairy cows fed aflatoxin-contaminated diets. J. Anim. Physiol. Anim. Nutr. (Berl.) 2013, 97, 694–700. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission regulation 2013/1060/EC of 29 October 2013 concerning the authorisation of bentonite as a feed additive for all animal species. Off. J. Eur. Commun. 2013, L289, 33–37. [Google Scholar]
- Applebaum, R.S.; Marth, E.H. Use of sulfite or bentonite to eliminate aflatoxin M1 from naturally contaminated raw milk. Z. Lebensm. Unters Forsch. 1982, 174, 303–305. [Google Scholar] [CrossRef]
- Carraro, A.; de Giacomo, A.; Giannossi, M.L.; Medici, L.; Muscarella, M.; Palazzo, L.; Quaranta, V.; Summa, V.; Tateo, F. Clay minerals as adsorbents of aflatoxin M1 from contaminated milk and effects on milk quality. Appl. Clay Sci. 2014, 88–89, 92–99. [Google Scholar] [CrossRef]
- El-Nezami, H.; Kankaanpaa, P.; Salminen, S.; Ahokas, J. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen, aflatoxin B1. Food Chem. Toxicol. 1998, 36, 321–326. [Google Scholar] [CrossRef]
- El-Nezami, H.; Kankaanpaa, P.; Salminen, S.; Ahokas, J. Physicochemical alterations enhance the ability of dairy strains of lactic acid bacteria to remove aflatoxin from contaminated media. J. Food Prot. 1998, 61, 466–468. [Google Scholar] [PubMed]
- Peltonen, K.; El-Nezami, H.; Haskard, C.; Ahokas, J.; Salminen, S. Aflatoxin B1 binding by dairy strains of lactic acid bacteria and bifidobacteria. J. Dairy Sci. 2001, 84, 2152–2156. [Google Scholar] [CrossRef]
- Hernandez-Mendoza, A.; Garcia, H.S.; Steele, J.L. Screening of Lactobacillus casei strains for their ability to bind aflatoxin B1. Food Chem. Toxicol. 2009, 47, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Khoury, A.E.; Atoui, A.; Yaghi, J. Analysis of aflatoxin M1 in milk and yogurt and AFM1 reduction by lactic acid bacteria used in lebanese industry. Food Control 2011, 22, 1695–1699. [Google Scholar] [CrossRef]
- Ayoub, M.M.; Sobeih, A.M.K.; Raslan, A.A. Evaluation of aflatoxin M1 in raw, processed milk and some milk products in Cairo with special reference to its recovery. Researcher 2011, 3, 5–11. [Google Scholar]
- Sarimehmetoglu, B.; Kuplulu, O. Binding ability of aflatoxin M1 to yoghurt bacteria. Ankara Univ. Vet. Fak. Derg. 2004, 51, 195–198. [Google Scholar]
- Elsanhoty, R.M.; Salam, S.A.; Ramadan, M.F.; Badr, F.H. Detoxification of aflatoxin M1 in yoghurt using probiotics and lactic acid bacteria. Food Control 2014, 43, 129–134. [Google Scholar] [CrossRef]
- Serrano-Niño, J.C.; Cavazos-Garduño, A.; Hernandez-Mendoza, A.; Applegate, B.; Ferruzzi, M.G.; Martin-González, M.F.S.; García, H.S. Assessment of probiotic strains ability to reduce the bioaccessibility of aflatoxin M1 in artificially contaminated milk using an in vitro digestive model. Food Control 2013, 31, 202–207. [Google Scholar] [CrossRef]
- Kabak, B.; Var, I. Factors affecting the removal of aflatoxin M1 from food model by Lactobacillus and Bifidobacterium strains. J. Environ. Sci. Health B 2008, 43, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Bovo, F.; Corassin, C.; Rosim, R.; de Oliveira, C.F. Efficiency of lactic acid bacteria strains for decontamination of aflatoxin M1 in phosphate buffer saline solution and in skimmed milk. Food Bioprocess Technol. 2013, 6, 2230–2234. [Google Scholar] [CrossRef]
- Corassin, C.H.; Bovo, F.; Rosim, R.E.; Oliveira, C.A. Efficiency of Saccharomyces cerevisiae and lactic acid bacteria strains to bind aflatoxin M1 in UHT skim milk. Food Control 2013, 31, 80–83. [Google Scholar] [CrossRef]
- Nakazato, M.; Morozumi, S.; Saito, K.; Fujinuma, K.; Nishima, T.; Kasai, N. Interconversion of aflatoxin B1 and aflatoxicol by several fungi. Appl. Environ. Microbiol. 1990, 56, 1465–1470. [Google Scholar] [PubMed]
- Guan, S.; Zhou, T.; Yin, Y.L.; Xie, M.Y.; Ruan, Z.; Young, J.C. Microbial strategies to control aflatoxins in food and feed. World Mycotoxin J. 2011, 4, 413–424. [Google Scholar] [CrossRef]
- Huynh, V.L.; Gerdes, R.G.; Lloyd, A.B. Synthesis and degradation of aflatoxins by Aspergillus parasiticus. II. Comparative toxicity and mutagenicity of aflatoxin B1 and its autolytic breakdown products. Aust. J. Biol. Sci. 1984, 37, 123–129. [Google Scholar] [PubMed]
- Ciegler, A.; Lillehoj, E.B.; Peterson, R.E.; Hall, H.H. Microbial detoxification of aflatoxin. Appl. Microbiol. 1966, 14, 934–939. [Google Scholar] [PubMed]
- Hormisch, D.; Brost, I.; Kohring, G.W.; Giffhorn, F.; Kroppenstedt, R.M.; Stackebrandt, E.; Farber, P.; Holzapfel, W.H. Mycobacterium fluoranthenivorans sp. nov., a fluoranthene and aflatoxin B1 degrading bacterium from contaminated soil of a former coal gas plant. Syst. Appl. Microbiol. 2004, 27, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Alberts, J.F.; Engelbrecht, Y.; Steyn, P.S.; Holzapfel, W.H.; van Zyl, W.H. Biological degradation of aflatoxin B1 by Rhodococcus erythropolis cultures. Int. J. Food Microbiol. 2006, 109, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Ji, C.; Zhou, T.; Li, J.; Ma, Q.; Niu, T. Aflatoxin B1 degradation by Stenotrophomonas maltophilia and other microbes selected using coumarin medium. Int. J. Mol. Sci. 2008, 9, 1489–1503. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Zhao, L.; Ma, Q.; Zhou, T.; Wang, N.; Hu, X.; Ji, C. In vitro efficacy of Myxococcus fulvus ANSM068 to biotransform aflatoxin B1. Int. J. Mol. Sci. 2010, 11, 4063–4079. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.H.; Brackett, R.E. The influence of divalent cations and chelators on aflatoxin B1 degradation by Flavobacterium aurantiacum. J. Food Prot. 2000, 63, 102–105. [Google Scholar] [PubMed]
- Teniola, O.D.; Addo, P.A.; Brost, I.M.; Farber, P.; Jany, K.D.; Alberts, J.F.; van Zyl, W.H.; Steyn, P.S.; Holzapfel, W.H. Degradation of aflatoxin B1 by cell-free extracts of Rhodococcus erythropolis and Mycobacterium fluoranthenivorans sp. nov. DSM44556(T). Int. J. Food Microbiol. 2005, 105, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.L.; Yao, D.S.; Liang, Y.Q.; Zhou, T.H.; Song, Y.P.; Zhao, L.; Ma, L. Production, purification, and characterization of an intracellular aflatoxin-detoxifizyme from Armillariella tabescens (E-20). Food Chem. Toxicol. 2001, 39, 461–466. [Google Scholar] [CrossRef]
- Motomura, M.; Toyomasu, T.; Mizuno, K.; Shinozawa, T. Purification and characterization of an aflatoxin degradation enzyme from Pleurotus ostreatus. Microbiol. Res. 2003, 158, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.H.; Guan, S.; Gao, X.; Ma, Q.G.; Lei, Y.P.; Bai, X.M.; Ji, C. Preparation, purification and characteristics of an aflatoxin degradation enzyme from Myxococcus fulvus ANSM068. J. Appl. Microbiol. 2011, 110, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, D.; Mo, X.; Xie, C.; Yao, D. A fungal enzyme with the ability of aflatoxin B1 conversion: Purification and ESI-MS/MS identification. Microbiol. Res. 2011, 166, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Applebaum, R.S.; Brackett, R.E.; Wiseman, D.W.; Marth, E.H. Responses of dairy cows to dietary aflatoxin: Feed intake and yield, toxin content, and quality of milk of cows treated with pure and impure aflatoxin. J. Dairy Sci. 1982, 65, 1503–1508. [Google Scholar] [CrossRef]
- Polonelli, L.; Giovati, L.; Magliani, W.; Conti, S.; Sforza, S.; Calabretta, A.; Casoli, C.; Ronzi, P.; Grilli, E.; Gallo, A.; et al. Vaccination of lactating dairy cows for the prevention of aflatoxin B1 carry over in milk. PLoS ONE 2011. [Google Scholar] [CrossRef] [PubMed]
- Giovati, L.; Gallo, A.; Masoero, F.; Cerioli, C.; Ciociola, T.; Conti, S.; Magliani, W.; Polonelli, L. Vaccination of heifers with anaflatoxin improves the reduction of aflatoxin B1 carry over in milk of lactating dairy cows. PLoS ONE 2014. [Google Scholar] [CrossRef] [PubMed]
- Ueno, I.; Chu, F.S. Modification of hepatotoxic effects of aflatoxin B1 in rabbits by immunization. Experientia 1978, 34, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Odunola, O.A.; Uwaifo, A.O. Binding reaction of aflatoxin B1 with immunoglobulin G against aflatoxin B1-bovine serum albumin complex. Afr. J. Med. Med. Sci. 1998, 27, 1–4. [Google Scholar] [PubMed]
- Odunola, O.A.; Uwaifo, A.O. Immune response to aflatoxin B1-histone H1 complex. Afr. J. Med. Med. Sci. 2000, 29, 105–110. [Google Scholar] [PubMed]
- Wilkinson, J.; Rood, D.; Minior, D.; Guillard, K.; Darre, M.; Silbart, L.K. Immune response to a mucosally administered aflatoxin B1 vaccine. Poult. Sci. 2003, 82, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Caviezel, M.; Aeschbach, A.P.; Lutz, W.K.; Schlatter, C. Reduction of covalent binding of aflatoxin B1 to rabbit liver DNA after immunization against this carcinogen. Arch. Toxicol. Suppl. 1984, 7, 249–252. [Google Scholar] [PubMed]
- Sizaret, P.; Malaveille, C.; Brun, G.; Aguelon, A.M.; Toussaint, G. Inhibition by specific antibodies of the mutagenicity of aflatoxin B1 in bacteria. Oncodev. Biol. Med. 1982, 3, 125–134. [Google Scholar] [PubMed]
- Odugbesan, E.A.; Osowole, O.A.; Uwaifo, A.O. Effect of antiserum against aflatoxin B1-bovine serum albumin complex on aflatoxin B1-induced lysogenesis. Mutat. Res. 1988, 209, 7–11. [Google Scholar] [CrossRef]
- Prandini, A.; Tansini, G.; Sigolo, S.; Filippi, L.; Laporta, M.; Piva, G. On the occurrence of aflatoxin M1 in milk and dairy products. Food Chem. Toxicol. 2009, 47, 984–991. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovati, L.; Magliani, W.; Ciociola, T.; Santinoli, C.; Conti, S.; Polonelli, L. AFM1 in Milk: Physical, Biological, and Prophylactic Methods to Mitigate Contamination. Toxins 2015, 7, 4330-4349. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins7104330
Giovati L, Magliani W, Ciociola T, Santinoli C, Conti S, Polonelli L. AFM1 in Milk: Physical, Biological, and Prophylactic Methods to Mitigate Contamination. Toxins. 2015; 7(10):4330-4349. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins7104330
Chicago/Turabian StyleGiovati, Laura, Walter Magliani, Tecla Ciociola, Claudia Santinoli, Stefania Conti, and Luciano Polonelli. 2015. "AFM1 in Milk: Physical, Biological, and Prophylactic Methods to Mitigate Contamination" Toxins 7, no. 10: 4330-4349. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins7104330