Distribution and Metabolism of Bt-Cry1Ac Toxin in Tissues and Organs of the Cotton Bollworm, Helicoverpa armigera
Abstract
:1. Introduction
2. Results
2.1. Concentrations of Cry1Ac Toxin in H. armigera Bodies at Different Developmental Stages
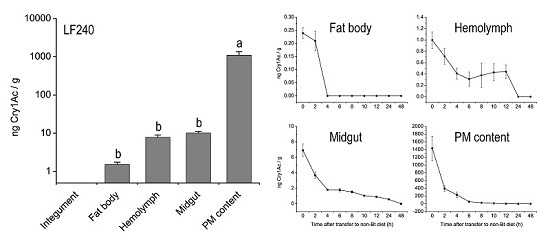
2.2. Tissue Distribution of Cry1Ac Toxin in H. armigera Larvae
2.3. Metabolic Status of Cry1Ac Toxin in H. armigera Larvae Tissues
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Insect Culture
5.2. Quantification of Cry1Ac in H. armigera Body at Different Developmental Stages
5.3. Quantification of Cry1Ac in H. armigera Larval Tissues
5.4. Metabolism of Cry1Ac in H. armigera Larval Tissues
5.5. Tissue Collection
5.6. Quantitative Enzyme-Linked Immunosorbent Assay (ELISA)
5.7. Western Blots
5.8. Data Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gill, S.S.; Cowles, E.A.; Pietrantonio, P.V. The mode of action of Bacillus thuringiensis endotoxins. Annu. Rev. Entomol. 1992, 37, 615–636. [Google Scholar] [CrossRef] [PubMed]
- Schnepf, E.; Crickmore, N.; Van Rie, J.; Lereclus, D.; Baum, J.; Feitelson, J.; Zeigler, D.R.; Dean, D.H. Bacillus thuringiensis and its pesticidal crystal toxins. Microbiol. Mol. Biol. Rev. 1998, 62, 775–806. [Google Scholar] [PubMed]
- Whalon, M.E.; Wingerd, B.A. Bt: Mode of action and use. Arch. Insect Biochem. Physiol. 2003, 54, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Sanahuja, G.; Banakar, R.; Twyman, R.M.; Capell, T.; Christou, P. Bacillus thuringiensis: A century of research, development and commercial applications. Plant Biotechnol. J. 2011, 9, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, N. Case studies: A hard look at GM crops. Nature 2013, 497, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.E. Peer-reviewed surveys indicate positive impact of commercialized GM crops. Nat. Biotechnol. 2010, 28, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.M.; Guo, Y.Y.; Lv, N.; Greenplate, J.T.; Deaton, R. Efficacy of transgenic cotton containing a Cry1Ac gene from Bacillus thuringiensis against Helicoverpa armigera (Lepidoptera: Noctuidae) in northern China. J. Econ. Entomol. 2003, 96, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.M.; Lu, Y.H.; Feng, H.Q.; Jiang, Y.Y.; Zhao, J.Z. Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin-containing cotton. Science 2008, 321, 1676–1678. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Wu, K.M.; Jiang, Y.Y.; Guo, Y.Y.; Desneux, N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 2012, 487, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Fitt, G.P. The ecology of Heliothis species in relation to agroecosystems. Annu. Rev. Entomol. 1989, 34, 17–52. [Google Scholar] [CrossRef]
- Bravo, A.; Gómez, I.; Conde, J.; Muñoz-Garay, C.; Sánchez, J.; Miranda, R.; Zhuang, M.; Gill, S.S.; Soberón, M. Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to insertion into membrane microdomains. Biochim. Biophys. Acta 2004, 1667, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Griffitts, J.S.; Haslam, S.M.; Yang, T.; Garczynski, S.F.; Mulloy, B.; Morris, H.; Cremer, P.S.; Dell, A.; Adang, M.J.; Aroian, R.V. Glycolipids as receptors for Bacillus thuringiensis crystal toxin. Science 2005, 307, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Wu, K.M.; Wu, Y.D.; Gao, Y.L.; Ning, C.M.; Oppert, B. Reduction of Bacillus thuringiensis Cry1Ac toxicity against Helicoverpa armigera by a soluble toxin-binding cadherin fragment. J. Insect Physiol. 2009, 55, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Arenas, I.; Bravo, A.; Soberón, M.; Gómez, I. Role of alkaline phosphatase from Manduca sexta in the mechanism of action of Bacillus thuringiensis Cry1Ab toxin. J. Biol. Chem. 2010, 285, 12497–12503. [Google Scholar] [CrossRef] [PubMed]
- Head, G.; Brown, C.R.; Groth, M.E.; Duan, J.J. Cry1Ab protein levels in phytophagous insects feeding on transgenic corn: Implications for secondary exposure risk assessment. Entomol. Exp. Appl. 2001, 99, 37–45. [Google Scholar] [CrossRef]
- Dutton, A.; Klein, H.; Romeis, J.; Bigler, F. Uptake of Bt-toxin by herbivores feeding on transgenic maize and consequences for the predator Chrysoperla carnea. Ecol. Entomol. 2002, 27, 441–447. [Google Scholar] [CrossRef]
- Knecht, S.; Nentwig, W. Effect of Bt maize on the reproduction and development of saprophagous Diptera over multiple generations. Basic Appl. Ecol. 2010, 11, 346–353. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; López, C.; Albajes, R.; Eizaguirre, M. Low susceptibility of non-target Lepidopteran maize pests to the Bt toxin Cry1Ab. Bull. Entomol. Res. 2012, 102, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Hedo, M.; Reiter, D.; López, C.; Eizaguirre, M. Processing of the maize Bt toxin in the gut of Mythimna unipuncta caterpillars. Entomol. Exp. Appl. 2013, 148, 56–64. [Google Scholar] [CrossRef]
- Vojtech, E.; Meissle, M.; Poppy, G.M. Effects of Bt maize on the herbivore Spodoptera littoralis (Lepidoptera: Noctuidae) and the parasitoid Cotesia marginiventris (Hymenoptera: Braconidae). Transgenic Res. 2005, 14, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Romeis, J. Bt maize expressing Cry3Bb1 does not harm the spider mite, Tetranychus urticae, or its ladybird beetle predator, Stethorus punctillum. Biol. Control 2010, 53, 337–344. [Google Scholar] [CrossRef]
- Wang, X.F.; Chen, X.Y.; Xu, J.F.; Dai, C.; Shen, W.B. Degradation and detection of transgenic Bacillus thuringiensis DNA and proteins in flour of three genetically modified rice events submitted to a set of thermal processes. Food Chem. Toxicol. 2015, 84, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Lightwood, D.J.; Ellar, D.J.; Jarrett, P. Role of proteolysis in determining potency of Bacillus thuringiensis Cry1Ac δ-endotoxin. Appl. Environ. Microbiol. 2000, 66, 5174–5181. [Google Scholar] [CrossRef] [PubMed]
- Rukmini, V.; Reddy, C.Y.; Venkateswerlu, G. Bacillus thuringiensis crystal δ-endotoxin: Role of proteases in the conversion of protoxin to toxin. Biochimie 2000, 82, 109–116. [Google Scholar] [CrossRef]
- Budatha, M.; Meur, G.; Dutta-Gupta, A. A novel aminopeptidase in the fat body of the moth Achaea janata as a receptor for Bacillus thuringiensis Cry toxins and its comparison with midgut aminopeptidase. Biochem. J. 2007, 405, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Budatha, M.; Meur, G.; Kirti, P.B.; Dutta-Gupta, A. Characterization of Bacillus thuringiensis Cry toxin binding novel GPI anchored aminopeptidase from fat body of the moth Spodoptera litura. Biotechnol. Lett. 2007, 29, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- Ningshen, T.J.; Aparoy, P.; Ventaku, V.R.; Dutta-Gupta, A. Functional interpretation of a non-gut hemocoelic tissue aminopeptidase N (APN) in a Lepidopteran insect pest Achaea janata. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.J. Too much of a good thing: How insects cope with excess ions or toxins in the diet. J. Exp. Biol. 2009, 212, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Beyenbach, K.W.; Skaer, H.; Dow, J.A. The developmental, molecular, and transport biology of malpighian tubules. Annu. Rev. Entomol. 2010, 55, 351–374. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.; Hendrickx, K.; Jansens, S.; Peferoen, M. Immunocytochemical analysis of specific binding of Bacillus thuringiensis insecticidal crystal proteins to Lepidopteran and Coleopteran midgut membranes. J. Invertebr. Pathol. 1992, 60, 247–253. [Google Scholar] [CrossRef]
- Rees, J.S.; Jarrett, P.; Ellar, D.J. Peritrophic membrane contribution to Bt Cry δ-endotoxin susceptibility in Lepidoptera and the effect of Calcofluor. J. Invertebr. Pathol. 2009, 100, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Valaitis, A.P.; Podgwaite, J.D. Bacillus thuringiensis Cry1A toxin-binding glycoconjugates present on the brush border membrane and in the peritrophic membrane of the Douglas-fir tussock moth are peritrophins. J. Invertebr. Pathol. 2013, 112, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dutra, C.C.; Koch, R.L.; Burkness, E.C.; Meissle, M.; Romeis, J.; Hutchison, W.D.; Fernandes, M.G. Harmonia axyridis (Coleoptera: Coccinellidae) exhibits no preference between Bt and non-Bt maize fed Spodoptera frugiperda (Lepidoptera: Noctuidae). PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Harwood, J.D.; Wallin, W.G.; Obrycki, J.J. Uptake of Bt endotoxins by nontarget herbivores and higher order arthropod predators: Molecular evidence from a transgenic corn agroecosystem. Mol. Ecol. 2005, 14, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.L.; Romeis, J.; Li, Y.H.; Li, X.J.; Wu, K.M. Acquisition of Cry1Ac protein by non-target arthropods in Bt soybean fields. PLoS ONE 2014, 9, e103973. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.S.; Zhang, Y.H.; Liu, P.; Xie, J.Q.; He, Y.Y.; Deng, C.S.; De Clercq, P.; Pang, H. Effects of transgenic Cry1Ac+CpTI cotton on non-target mealybug pest Ferrisia virgata and its predator Cryptolaemus montrouzieri. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.M.; Guo, Y.Y. Changes in susceptibility to conventional insecticides of a Cry1Ac-selected population of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Pest Manag. Sci. 2004, 60, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.M.; Wu, K.M.; Yu, H.K.; Li, K.K.; Feng, X.; Guo, Y.Y. Changes of inheritance mode and fitness in Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) along with its resistance evolution to Cry1Ac toxin. J. Invertebr. Pathol. 2008, 97, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.C.; Zhang, L.L.; Liang, G.M.; Li, X.C.; Wu, K.M. Involvement of nonbinding site toxinases in the development of resistance of Helicoverpa armigera (Lepidoptera: Noctuidae) to Cry1Ac. J. Econ. Entomol. 2013, 106, 2514–2521. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.C.; Feng, H.Q.; Guo, F.; Wu, K.M.; Li, X.C.; Liang, G.M.; Desneux, N. Quantitative analysis of fitness costs associated with the development of resistance to the Bt toxin Cry1Ac in Helicoverpa armigera. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.T.; Zhang, T.; Liu, C.X.; Heckel, D.G.; Li, X.C.; Tabashnik, B.E.; Wu, K.M. Mis-splicing of the ABCC2 gene linked with Bt toxin resistance in Helicoverpa armigera. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Li, Y.; Xiao, Y.; Ali, A.; Dhiloo, K.H.; Chen, W.; Wu, K. Distribution and Metabolism of Bt-Cry1Ac Toxin in Tissues and Organs of the Cotton Bollworm, Helicoverpa armigera. Toxins 2016, 8, 212. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins8070212
Zhao Z, Li Y, Xiao Y, Ali A, Dhiloo KH, Chen W, Wu K. Distribution and Metabolism of Bt-Cry1Ac Toxin in Tissues and Organs of the Cotton Bollworm, Helicoverpa armigera. Toxins. 2016; 8(7):212. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins8070212
Chicago/Turabian StyleZhao, Zhuoya, Yunhe Li, Yutao Xiao, Abid Ali, Khalid Hussain Dhiloo, Wenbo Chen, and Kongming Wu. 2016. "Distribution and Metabolism of Bt-Cry1Ac Toxin in Tissues and Organs of the Cotton Bollworm, Helicoverpa armigera" Toxins 8, no. 7: 212. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins8070212