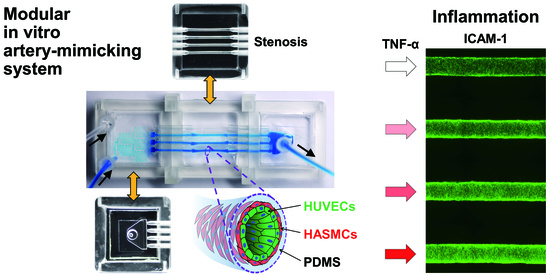
Modular 3D In Vitro Artery-Mimicking Multichannel System for Recapitulating Vascular Stenosis and Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of a Modular Device for In Vitro Artery Mimicking
2.3. HASMC–HUVEC Coculture in a Modular Device
2.4. Characterization of the Microfluidic CGG Module
2.5. TNF-α Treatment for the Inflammation Model
2.6. Monocytic THP-1 Cell Adhesion in the Inflammation Model
2.7. Immunofluorescence Staining and Imaging
2.8. Statistical Analysis
3. Results and Discussion
3.1. Fabrication of a Modular 3D In Vitro Artery-Mimicking Multichannel System
3.2. Concentration Gradient Generation in a Multichannel with a Microfluidic CGG Module
3.3. TNF-α Dose-Dependent Adhesion Molecule and vWF Expression in an Inflammation Model
3.4. TNF-α Dose-Dependent Monocyte Adhesion in the Inflammation Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Glezeva, N.; Baugh, J.A. Role of inflammation in the pathogenesis of heart failure with preserved ejection fraction and its potential as a therapeutic target. Heart Fail. Rev. 2014, 19, 681–694. [Google Scholar] [CrossRef]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [Green Version]
- Inzitari, D.; Eliasziw, M.; Gates, P.; Sharpe, B.L.; Chan, R.K.T.; Meldrum, H.E.; Barnett, H.J.M. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. N. Engl. J. Med. 2000, 342, 1693–1701. [Google Scholar] [CrossRef]
- Truskey, G.A. Endothelial cell vascular smooth muscle cell co-culture assay for high throughput screening assays for discovery of anti-angiogenesis agents and other therapeutic molecules. Int. J. High Throughput Screen. 2010, 2010, 171. [Google Scholar] [CrossRef] [Green Version]
- Inamdar, N.K.; Borenstein, J.T. Microfluidic cell culture models for tissue engineering. Curr. Opin. Biotechnol. 2011, 22, 681–689. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Chung, M.; Jeon, N.L. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 2013, 13, 1489–1500. [Google Scholar] [CrossRef]
- Tsai, M.; Kita, A.; Leach, J.; Rounsevell, R.; Huang, J.N.; Moake, J.; Ware, R.E.; Fletcher, D.A.; Lam, W.A. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J. Clin. Investig. 2011, 122, 408–418. [Google Scholar] [CrossRef] [Green Version]
- Giridharan, G.A.; Nguyen, M.-D.; Estrada, R.; Parichehreh, V.; Hamid, T.; Ismahil, M.A.; Prabhu, S.D.; Sethu, P. Microfluidic cardiac cell culture model (μCCCM). Anal. Chem. 2010, 82, 7581–7587. [Google Scholar] [CrossRef]
- Goral, V.N.; Hsieh, Y.-C.; Petzold, O.N.; Clark, J.S.; Yuen, P.K.; Faris, R.A. Perfusion-based microfluidic device for three-dimensional dynamic primary human hepatocyte cell culture in the absence of biological or synthetic matrices or coagulants. Lab Chip 2010, 10, 3380–3386. [Google Scholar] [CrossRef]
- Domansky, K.; Inman, W.; Serdy, J.; Dash, A.; Lim, M.H.; Griffith, L.G. Perfused multiwell plate for 3D liver tissue engineering. Lab Chip 2010, 10, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [Green Version]
- Sung, J.H.; Yu, J.; Luo, D.; Shuler, M.L.; March, J.C. Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab Chip 2011, 11, 389–392. [Google Scholar] [CrossRef]
- Li, M.; Qian, M.; Kyler, K.; Xu, J. Endothelial–vascular smooth muscle cells interactions in atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 151. [Google Scholar] [CrossRef] [Green Version]
- Oosterhoff, L.A.; Kruitwagen, H.S.; van Wolferen, M.E.; Van Balkom, B.W.; Mokry, M.; Lansu, N.; van den Dungen, N.A.; Penning, L.C.; Spanjersberg, T.C.; de Graaf, J.W.; et al. Characterization of endothelial and smooth muscle cells from different canine vessels. Front. Physiol. 2019, 10, 101. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.Y.; Yeom, E. Microfluidic measurement for blood flow and platelet adhesion around a stenotic channel: Effects of tile size on the detection of platelet adhesion in a correlation map. Biomicrofluidics 2017, 11, 024119. [Google Scholar] [CrossRef]
- Ha, H.; Lee, S.-J. Hemodynamic features and platelet aggregation in a stenosed microchannel. Microvasc. Res. 2013, 90, 96–105. [Google Scholar] [CrossRef]
- Li, M.; Hotaling, N.A.; Ku, D.N.; Forest, C.R. Microfluidic thrombosis under multiple shear rates and antiplatelet therapy doses. PLoS ONE 2014, 9, e82493. [Google Scholar] [CrossRef] [PubMed]
- Menon, N.V.; Tay, H.M.; Wee, S.N.; Li, K.H.H.; Hou, H.W. Micro-engineered perfusable 3D vasculatures for cardiovascular diseases. Lab Chip 2017, 17, 2960–2968. [Google Scholar] [CrossRef] [PubMed]
- Mannino, R.G.; Myers, D.R.; Ahn, B.; Wang, Y.; Rollins, M.; Gole, H.; Lin, A.S.; Guldberg, R.E.; Giddens, D.P.; Timmins, L.H.; et al. Do-it-yourself in vitro vasculature that recapitulates in vivo geometries for investigating endothelial-blood cell interactions. Sci. Rep. 2015, 5, 12401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, A.; Daniel Ou-Yang, H.; Lowe-Krentz, L.; Muzykantov, V.R.; Liu, Y. Biomimetic channel modeling local vascular dynamics of pro-inflammatory endothelial changes. Biomicrofluidics 2016, 10, 014101. [Google Scholar] [CrossRef] [Green Version]
- van Dijk, C.G.M.; Brandt, M.M.; Poulis, N.; Anten, J.; van der Moolen, M.; Kramer, L.; Homburg, E.F.G.A.; Louzao-Martinez, L.; Pei, J.; Krebber, M.M.; et al. A new microfluidic model that allows monitoring of complex vascular structures and cell interactions in a 3D biological matrix. Lab Chip 2020, 20, 1827–1844. [Google Scholar] [CrossRef]
- Menon, N.V.; Tay, H.M.; Pang, K.T.; Dalan, R.; Wong, S.C.; Wang, X.; Li, K.H.H.; Hou, H.W. A tunable microfluidic 3D stenosis model to study leukocyte-endothelial interactions in atherosclerosis. APL Bioeng. 2018, 2, 016103. [Google Scholar] [CrossRef]
- Menon, N.V.; Su, C.; Pang, K.T.; Phua, Z.J.; Tay, H.M.; Dalan, R.; Wang, X.; Li, K.H.H.; Hou, H.W. Recapitulating atherogenic flow disturbances and vascular inflammation in a perfusable 3D stenosis model. Biofabrication 2020, 12, 045009. [Google Scholar] [CrossRef]
- Cho, M.; Park, J.-K. Fabrication of a Perfusable 3D In Vitro Artery-Mimicking Multichannel System for Artery Disease Models. ACS Biomater. Sci. Eng. 2020, 6, 5326–5336. [Google Scholar] [CrossRef]
- Urschel, K.; Cicha, I. TNF-α in the cardiovascular system: From physiology to therapy. Int. J. Interferon Cytokine Mediat. Res. 2015, 7, 9–25. [Google Scholar]
- Ott, L.W.; Resing, K.A.; Sizemore, A.W.; Heyen, J.W.; Cocklin, R.R.; Pedrick, N.M.; Woods, H.C.; Chen, J.Y.; Goebl, M.G.; Witzmann, F.A.; et al. Tumor necrosis factor-α-and interleukin-1-induced cellular responses: Coupling proteomic and genomic information. J. Proteome Res. 2007, 6, 2176–2185. [Google Scholar] [CrossRef] [Green Version]
- Holbrook, J.; Lara-Reyna, S.; Jarosz-Griffiths, H.; McDermott, M.F. Tumour necrosis factor signalling in health and disease. F1000Research 2019, 8, 111. [Google Scholar] [CrossRef]
- Sawa, Y.; Sugimoto, Y.; Ueki, T.; Ishikawa, H.; Sato, A.; Nagato, T.; Yoshida, S. Effects of TNF-α on leukocyte adhesion molecule expressions in cultured human lymphatic endothelium. J. Histochem. Cytochem. 2007, 55, 721–733. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.J.; Muller, S.; Wang, X.; Zhuang, F.Y.; Stoltz, J.F. Regulation of von willebrand factor of human endothelial cells exposed to laminar flows: An in vitro study. Clin. Hemorheol. Microcirc. 2000, 23, 1–11. [Google Scholar]
- Chiu, J.-J.; Chien, S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol. Rev. 2011, 91, 327–387. [Google Scholar] [CrossRef] [Green Version]
- Chiu, J.J.; Wung, B.S.; Shyy, J.Y.J.; Hsieh, H.J.; Wang, D.L. Reactive oxygen species are involved in shear stress-induced intercellular adhesion molecule-1 expression in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 3570–3577. [Google Scholar] [CrossRef]
- Jilkova, Z.M.; Lisowska, J.; Manet, S.; Verdier, C.; Deplano, V.; Geindreau, C.; Faurobert, E.; Albigès-Rizo, C.; Duperray, A. CCM proteins control endothelial β1 integrin dependent response to shear stress. Biol. Open 2014, 3, 1228–1235. [Google Scholar] [CrossRef] [Green Version]
- Tsuboi, H.; Ando, J.; Korenaga, R.; Takada, Y.; Kamiya, A. Flow stimulates ICAM-1 expression time and shear stress dependently in cultured human endothelial cells. Biochem. Biophys. Res. Commun. 1995, 206, 988–996. [Google Scholar] [CrossRef]
- Nagel, T.; Resnick, N.; Atkinson, W.J.; Dewey, C.F.; Gimbrone, M.A. Shear stress selectively upregulates intercellular adhesion molecule-1 expression in cultured human vascular endothelial cells. J. Clin. Investig. 1994, 94, 885–891. [Google Scholar] [CrossRef] [Green Version]
- Chiu, J.-J.; Lee, P.-L.; Chen, C.-N.; Lee, C.-I.; Chang, S.-F.; Chen, L.-J.; Lien, S.-C.; Ko, Y.-C.; Usami, S.; Chien, S. Shear stress increases ICAM-1 and decreases VCAM-1 and E-selectin expressions induced by tumor necrosis factor-α in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 73–79. [Google Scholar] [CrossRef]
- Galbusera, M.; Zoja, C.; Donadelli, R.; Paris, S.; Morigi, M.; Benigni, A.; Figliuzzi, M.; Remuzzi, G.; Remuzzi, A. Fluid shear stress modulates von Willebrand factor release from human vascular endothelium. Blood 1997, 90, 1558–1564. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, K.; Morrell, C.N.; Lowenstein, C.J. Sphingosine 1-phosphate activates Weibel-Palade body exocytosis. Proc. Natl. Acad. Sci. USA 2004, 101, 11483–11487. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, L.; Dong, F.; Guo, L.; Hou, Y.; Hu, H.; Yan, S.; Zhou, X.; Liao, L.; Allen, T.D.; et al. Plasma von Willebrand factor level is transiently elevated in a rat model of acute myocardial infarction. Exp. Ther. Med. 2015, 10, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Rainger, G.E.; Nash, G.B. Cellular pathology of atherosclerosis: Smooth muscle cells prime cocultured endothelial cells for enhanced leukocyte adhesion. Circ. Res. 2001, 88, 615–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Tang, M.; Huang, D.; Jiang, W.; Li, M.; Ji, H.; Park, J.; Xu, B.; Atchison, L.J.; Truskey, G.A.; et al. Real-time observation of leukocyte–endothelium interactions in tissue-engineered blood vessel. Lab Chip 2018, 18, 2047–2054. [Google Scholar] [CrossRef] [PubMed]
- Čejková, S.; Králová-Lesná, I.; Poledne, R. Monocyte adhesion to the endothelium is an initial stage of atherosclerosis development. Cor Vasa 2016, 58, e419–e425. [Google Scholar] [CrossRef]
- Mestas, J.; Ley, K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc. Med. 2008, 18, 228–232. [Google Scholar] [CrossRef] [Green Version]
- Herbin, O.; Regelmann, A.G.; Ramkhelawon, B.; Weinstein, E.G.; Moore, K.J.; Alexandropoulos, K. Monocyte adhesion and plaque recruitment during atherosclerosis development is regulated by the adapter protein Chat-H/SHEP1. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1791–1801. [Google Scholar] [CrossRef] [Green Version]
- Srigunapalan, S.; Lam, C.; Wheeler, A.R.; Simmons, C.A. A microfluidic membrane device to mimic critical components of the vascular microenvironment. Biomicrofluidics 2011, 5, 013409. [Google Scholar] [CrossRef] [Green Version]
- Yin, W.; Shanmugavelayudam, S.K.; Rubenstein, D.A. The effect of physiologically relevant dynamic shear stress on platelet and endothelial cell activation. Thromb. Res. 2011, 127, 235–241. [Google Scholar] [CrossRef]
- Meza, D.; Musmacker, B.; Steadman, E.; Stransky, T.; Rubenstein, D.A.; Yin, W. Endothelial cell biomechanical responses are dependent on both fluid shear stress and tensile strain. Cell. Mol. Bioeng. 2019, 12, 311–325. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, M.; Park, J.-K. Modular 3D In Vitro Artery-Mimicking Multichannel System for Recapitulating Vascular Stenosis and Inflammation. Micromachines 2021, 12, 1528. https://0-doi-org.brum.beds.ac.uk/10.3390/mi12121528
Cho M, Park J-K. Modular 3D In Vitro Artery-Mimicking Multichannel System for Recapitulating Vascular Stenosis and Inflammation. Micromachines. 2021; 12(12):1528. https://0-doi-org.brum.beds.ac.uk/10.3390/mi12121528
Chicago/Turabian StyleCho, Minkyung, and Je-Kyun Park. 2021. "Modular 3D In Vitro Artery-Mimicking Multichannel System for Recapitulating Vascular Stenosis and Inflammation" Micromachines 12, no. 12: 1528. https://0-doi-org.brum.beds.ac.uk/10.3390/mi12121528