Numerical Analysis of Thermophoresis of a Charged Spheroidal Colloid in Aqueous Media
Abstract
:1. Introduction
2. Mathematical Model
2.1. Governing Equations
2.2. Boundary Conditions
2.3. Numerical Method
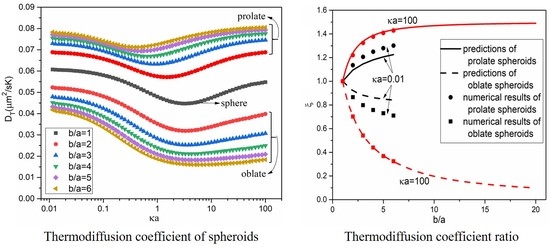
3. Results and Discussion
3.1. Flow Field Around Spheroidal Particles
3.2. Thermodiffusion Coefficient of Spheroidal Particles
3.3. Thermodiffusion Coefficient Ratio of Spheroids to Spheres
3.3.1. Thermodiffusion Coefficient Ratio for the Extremely Thin EDL Case
3.3.2. Thermodiffusion Coefficient Ratio for the Extremely Thick EDL Case
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tsuji, T.; Iseki, H.; Hanasaki, I.; Kawano, S. Negative thermophoresis of nanoparticles interacting with fluids through a purely-repulsive potential. J. Phys. Condens. Matter 2017, 29, 475101. [Google Scholar] [CrossRef]
- Fayolle, S.; Bickel, T.; Würger, A. Thermophoresis of charged colloidal particles. Phys. Rev. E 2008, 77 Pt 1, 041404. [Google Scholar] [CrossRef] [Green Version]
- Gittus, O.R.; Olarte-Plata, J.D.; Bresme, F. Thermal orientation and thermophoresis of anisotropic colloids: The role of the internal composition. Eur. Phys. J. E 2019, 42, 90. [Google Scholar] [CrossRef]
- Errarte, A.; Martin-Mayor, A.; Aginagalde, M.; Iloro, I.; Gonzalez, E.; Falcon-Perez, J.M.; Elortza, F.; Bou-Ali, M.M. Thermophoresis as a technique for separation of nanoparticle species in microfluidic devices. Int. J. Therm. Sci. 2020, 156, 106435. [Google Scholar] [CrossRef]
- Errarte, A.; Bou-Ali, M.M.; Aginagalde, M.; Santamaría, C. Thermodiffusion coefficients of nanofluid binary mixtures. Microgravity Sci. Technol. 2019, 31, 877–882. [Google Scholar] [CrossRef]
- Ramachandran, S.; Sobhan, C.B.; Peterson, G.P. Thermophoresis of nanoparticles in liquids. Int. J. Heat Mass Transf. 2020, 147, 118925. [Google Scholar] [CrossRef]
- Das, A.K.; Chatterjee, S. Analysis of thermophoresis and Brownian motion effect in heat transfer for nanofluid immersed distribution transformer. Electr. Eng. 2018, 100, 1963–1974. [Google Scholar] [CrossRef]
- Niether, D.; Wiegand, S. Thermophoresis of biological and biocompatible compounds in aqueous solution. J. Phys. Condens. Matter 2019, 31, 503003. [Google Scholar] [CrossRef]
- Niether, D.; Kawaguchi, T.; Hovancová, J.; Eguchi, K.; Dhont, J.K.G.; Kita, R.; Wiegand, S. Role of hydrogen bonding of cyclodextrin–drug complexes probed by thermodiffusion. Langmuir 2017, 33, 8483–8492. [Google Scholar] [CrossRef] [PubMed]
- Jacob, D.; Thuring, K.; Galliot, A.; Marchand, V.; Galvanin, A.; Ciftci, A.; Scharmann, K.; Stock, M.; Roignant, J.Y.; Leidel, S.A.; et al. Absolute quantification of noncoding RNA by microscale thermophoresis. Angew. Chem. Int. Ed. 2019, 58, 9565–9569. [Google Scholar] [CrossRef] [PubMed]
- Niether, D.; Sarter, M.; Koenig, B.W.; Fitter, J.; Stadler, A.M.; Wiegand, S. Thermophoresis: The case of streptavidin and Biotin. Polymers 2020, 12, 376. [Google Scholar] [CrossRef] [Green Version]
- Luettmer-Strathmann, J. Configurational contribution to the Soret effect of a protein ligand system: An investigation with density-of-states simulations. Eur. Phys. J. E 2019, 42, 77. [Google Scholar] [CrossRef] [PubMed]
- Ly, A.; Wurger, A. Hydrodynamic interactions in DNA thermophoresis. Soft Matter 2018, 14, 848–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Zhao, C.; Li, K.; Yang, C. Numerical analysis of thermal conductivity effect on thermophoresis of a charged colloidal particle in aqueous media. Int. J. Heat Mass Transf. 2019, 142, 118421. [Google Scholar] [CrossRef]
- Burelbach, J.; Frenkel, D.; Pagonabarraga, I.; Eiser, E. A unified description of colloidal thermophoresis. Eur. Phys. J. E 2018, 41, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, T.; Kozai, K.; Ishino, H.; Kawano, S. Direct observations of thermophoresis in microfluidic systems. Micro Nano Lett. 2017, 12, 520–525. [Google Scholar] [CrossRef] [Green Version]
- Kolacz, J.; Konya, A.; Selinger, R.L.B.; Wei, Q.H. Thermophoresis of colloids in nematic liquid crystal. Soft Matter 2020, 16, 1989–1995. [Google Scholar] [CrossRef] [PubMed]
- Makihara, T.; Demers, S.M.E.; Cole, L.E.D.; Zhang, A.; Hafner, J.H. Thermophoresis of gold nanorods from surface enhanced Raman scattering and real-time Rayleigh scattering in solution. Anal. Methods 2019, 11, 2482–2488. [Google Scholar] [CrossRef]
- Braibanti, M.; Vigolo, D.; Piazza, R. Does thermophoretic mobility depend on particle size? Phys. Rev. Lett. 2008, 100, 108303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichl, M.; Herzog, M.; Gotz, A.; Braun, D. Why charged molecules move across a temperature gradient: The role of electric fields. Phys. Rev. Lett. 2014, 112, 198101. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, C.; Lam, Y.C.; Huang, X. Thermophoresis of charged colloidal particles in aqueous media – Effect of particle size. Int. J. Heat Mass Tran. 2016, 101, 1283–1291. [Google Scholar] [CrossRef]
- Syshchyk, O.; Afanasenkau, D.; Wang, Z.; Kriegs, H.; Buitenhuis, J.; Wiegand, S. Influence of temperature and charge effects on thermophoresis of polystyrene beads. Eur. Phys. J. E 2016, 39, 129. [Google Scholar] [CrossRef] [PubMed]
- Iacopini, S.; Piazza, R. Thermophoresis in protein solutions. Europhys. Lett. 2003, 63, 247–253. [Google Scholar] [CrossRef]
- Eslahian, K.A.; Majee, A.; Maskos, M.; Wurger, A. Specific salt effects on thermophoresis of charged colloids. Soft Matter 2014, 10, 1931–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iacopini, S.; Rusconi, R.; Piazza, R. The "macromolecular tourist": Universal temperature dependence of thermal diffusion in aqueous colloidal suspensions. Eur. Phys. J. E 2006, 19, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.-Y.; Zhou, Y.; Yang, C.; Cao, B.-Y. Translational thermophoresis and rotational movement of peanut-like colloids under temperature gradient. Microfluid. Nanofluid 2015, 19, 805–811. [Google Scholar] [CrossRef]
- Blanco, P.; Kriegs, H.; Lettinga, M.P.; Holmqvist, P.; Wiegand, S. Thermal diffusion of a stiff rod-like mutant Y21M fd-virus. Biomacromolecules 2011, 12, 1602–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Z.; Yang, M.; Ripoll, M. Anisotropic thermophoresis. Soft Matter 2017, 13, 7283–7291. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Kriegs, H.; Buitenhuis, J.; Dhont, J.K.G.; Wiegand, S. Thermophoresis of charged colloidal rods. Soft Matter 2013, 9, 8697. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Niether, D.; Buitenhuis, J.; Liu, Y.; Lang, P.R.; Dhont, J.K.G.; Wiegand, S. Thermophoresis of a colloidal Rod: Contributions of charge and grafted polymers. Langmuir 2019, 35, 1000–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhont, J.K.; Briels, W.J. Single-particle thermal diffusion of charged colloids: Double-layer theory in a temperature gradient. Eur. Phys. J. E 2008, 25, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Keh, H.J.; Chang, Y.C. Thermophoresis of an aerosol spheroid along its axis of revolution. Phys. Fluids 2009, 21. [Google Scholar] [CrossRef]
- Gagnon, Z.; Gordon, J.; Sengupta, S.; Chang, H.-C. Bovine red blood cell starvation age discrimination through a glutaraldehyde-amplified dielectrophoretic approach with buffer selection and membrane cross-linking. Electrophoresis 2008, 29, 2272–2279. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, C.; He, J.; Zhou, Y.; Yang, C. Collective effects on thermophoresis of colloids: A microfluidic study within the framework of DLVO theory. Soft Matter 2013, 9, 7726. [Google Scholar] [CrossRef]
- Rasuli, S.N.; Golestanian, R. Soret motion of a charged spherical colloid. Phys. Rev. Lett. 2008, 101, 108301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wurger, A. Transport in charged colloids driven by thermoelectricity. Phys. Rev. Lett. 2008, 101, 108302. [Google Scholar] [CrossRef] [Green Version]
- Masliyah, J.H.; Bhattacharjee, S. Electrokinetic and Colloid Transport Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Leong, K.H. Thermophoresis and diffusiophoresis of large aerosol particles of different shapes. J. Aerosol Sci. 1984, 15, 511–517. [Google Scholar] [CrossRef]
- Datta, S.; Srivastava, D.K. Stokes drag on axially symmetric bodies: A new approach. Proc. Indian Acad. Sci. (Math. Sci.) 1999, 109, 441–452. [Google Scholar] [CrossRef]
- Jiang, H.R.; Yoshinaga, N.; Sano, M. Active motion of a Janus particle by self-thermophoresis in a defocused laser beam. Phys. Rev. Lett. 2010, 105, 268302. [Google Scholar] [CrossRef]
- Golestanian, R.; Liverpool, T.B.; Ajdari, A. Designing phoretic micro- and nano-swimmers. New J. Phys. 2007, 9, 126. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Yang, Y.; Zhu, C.; Yang, M.; Hu, Y. Numerical Analysis of Thermophoresis of a Charged Spheroidal Colloid in Aqueous Media. Micromachines 2021, 12, 224. https://0-doi-org.brum.beds.ac.uk/10.3390/mi12020224
Zhou Y, Yang Y, Zhu C, Yang M, Hu Y. Numerical Analysis of Thermophoresis of a Charged Spheroidal Colloid in Aqueous Media. Micromachines. 2021; 12(2):224. https://0-doi-org.brum.beds.ac.uk/10.3390/mi12020224
Chicago/Turabian StyleZhou, Yi, Yang Yang, Changxing Zhu, Mingyuan Yang, and Yi Hu. 2021. "Numerical Analysis of Thermophoresis of a Charged Spheroidal Colloid in Aqueous Media" Micromachines 12, no. 2: 224. https://0-doi-org.brum.beds.ac.uk/10.3390/mi12020224