Association of Common Variants of TNFSF13 and TNFRSF13B Genes with CLL Risk and Clinical Picture, as Well as Expression of Their Products—APRIL and TACI Molecules
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Copy Number Variations
2.2. Genotyping Results
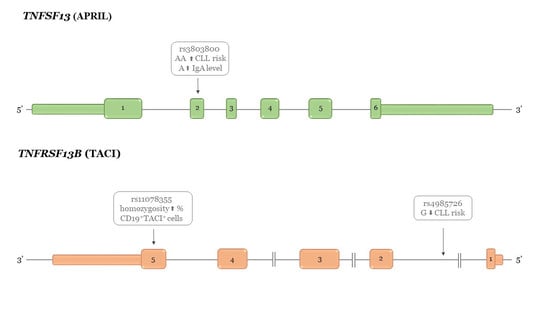
2.2.1. TNFSF13 Genetic Variants and Susceptibility to CLL
2.2.2. TNFRSF13B Genetic Variants and Susceptibility to CLL
2.3. Relationship between TNFSF13 Genetic Variants and APRIL Intracellular Expression in CLL Cells
2.4. Relationship between TNFSF13 Genetic Variants, Soluble APRIL, Rai Stage, and IgA Levels
2.5. Relationship between TNFRSF13B Genetic Variants and TACI Expression and Rai Stage
2.6. Prediction of Functional Effects for: TNFSF13 rs3803800G>A, rs4968210G>A and TNFRSF13B rs4985726C>G, and rs11078355A>G
3. Discussion
4. Materials and Methods
4.1. Study Groups
4.2. Selection of Single Nucleotide Polymorphisms and Genotyping
4.3. In Silico Analysis
- Ensembl Genome Browser (https://www.ensembl.org/Homo_sapiens/Info/Index);
- Human Splicing Finder (http://www.umd.be/HSF/ [40].
4.4. Copy Number Variations Determination
4.5. Detection of sAPRIL Plasma Level and Intracellular Expression of APRIL in CLL Patients
4.6. Detection of TACI Receptor Expression
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghamlouch, H.; Nguyen-Khac, F.; Bernard, O.A. Chronic lymphocytic leukaemia genomics and the precision medicine era. Br. J. Haematol. 2017, 178, 852–870. [Google Scholar] [CrossRef] [Green Version]
- Kipps, T.J.; Stevenson, F.K.; Wu, C.J.; Croce, C.M.; Packham, G.; Wierda, W.G.; O’Brien, S.; Gribben, J.; Rai, K. Chronic lymphocytic leukaemia. Nat. Rev. Dis. Primers 2017, 3, 17008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallek, M. Chronic lymphocytic leukemia: 2017 update on diagnosis, risk stratification, and treatment. Am. J. Hematol. 2017, 92, 946–965. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, G.; Montserrat, E. Critical molecular pathways in CLL therapy. Mol. Med. 2018, 24, 9. [Google Scholar] [CrossRef] [PubMed]
- Scarfo, L.; Ferreri, A.J.; Ghia, P. Chronic lymphocytic leukaemia. Crit. Rev. Oncol. Hematol. 2016, 104, 169–182. [Google Scholar] [CrossRef]
- Hallek, M.; Shanafelt, T.D.; Eichhorst, B. Chronic lymphocytic leukaemia. Lancet 2018, 391, 1524–1537. [Google Scholar] [CrossRef]
- Cerhan, J.R.; Slager, S.L. Familial predisposition and genetic risk factors for lymphoma. Blood 2015, 126, 2265–2273. [Google Scholar] [CrossRef]
- Speedy, H.E.; Beekman, R.; Chapaprieta, V.; Orlando, G.; Law, P.J.; Martin-Garcia, D.; Gutierrez-Abril, J.; Catovsky, D.; Bea, S.; Clot, G.; et al. Insight into genetic predisposition to chronic lymphocytic leukemia from integrative epigenomics. Nat. Commun. 2019, 10, 3615. [Google Scholar] [CrossRef]
- Kipps, T.J.; Choi, M.Y. Targeted Therapy in Chronic Lymphocytic Leukemia. Cancer J. 2019, 25, 378–385. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chaturvedi, N.K.; Rai, K.J.; Gilling-Cutucache, C.E.; Nordgren, T.M.; Moragues, M.; Lu, R.; Opavsky, R.; Bociek, G.R.; Weisenburger, D.D.; et al. Chronic lymphocytic leukemia cells in a lymph node microenvironment depict molecular signature associated with an aggressive disease. Mol. Med. 2014, 20, 290–301. [Google Scholar] [CrossRef]
- Mackay, F.; Schneider, P. TACI, an enigmatic BAFF/APRIL receptor, with new unappreciated biochemical and biological properties. Cytokine Growth Factor Rev. 2008, 19, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Vincent, F.B.; Saulep-Easton, D.; Figgett, W.A.; Fairfax, K.A.; Mackay, F. The BAFF/APRIL system: Emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev. 2013, 24, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Jasek, M.; Wagner, M.; Sobczynski, M.; Wolowiec, D.; Kuliczkowski, K.; Woszczyk, D.; Kielbinski, M.; Kusnierczyk, P.; Frydecka, I.; Karabon, L. Polymorphisms in genes of the BAFF/APRIL system may constitute risk factors of B-CLL--a preliminary study on a Polish population. Tissue Antigens 2015, 86, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Jasek, M.; Bojarska-Junak, A.; Wagner, M.; Sobczynski, M.; Wolowiec, D.; Rolinski, J.; Karabon, L.; Kusnierczyk, P. Association of variants in BAFF (rs9514828 and rs1041569) and BAFF-R (rs61756766) genes with the risk of chronic lymphocytic leukemia. Tumor Biol. 2016, 37, 13617–13626. [Google Scholar] [CrossRef] [Green Version]
- Guadagnoli, M.; Kimberley, F.C.; Phan, U.; Cameron, K.; Vink, P.M.; Rodermond, H.; Eldering, E.; Kater, A.P.; van Eenennaam, H.; Medema, J.P. Development and characterization of APRIL antagonistic monoclonal antibodies for treatment of B-cell lymphomas. Blood 2011, 117, 6856–6865. [Google Scholar] [CrossRef] [Green Version]
- Kimberley, F.C.; Hahne, M.; Medema, J.P. “APRIL hath put a spring of youth in everything”: Relevance of APRIL for survival. J. Cell Physiol. 2009, 218, 1–8. [Google Scholar] [CrossRef]
- Bojarska-Junak, A.; Hus, I.; Chocholska, S.; Wasik-Szczepanek, E.; Sieklucka, M.; Dmoszynska, A.; Rolinski, J. BAFF and APRIL expression in B-cell chronic lymphocytic leukemia: Correlation with biological and clinical features. Leuk. Res. 2009, 33, 1319–1327. [Google Scholar] [CrossRef]
- Kern, C.; Cornuel, J.F.; Billard, C.; Tang, R.; Rouillard, D.; Stenou, V.; Defrance, T.; Ajchenbaum-Cymbalista, F.; Simonin, P.Y.; Feldblum, S.; et al. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood 2004, 103, 679–688. [Google Scholar] [CrossRef] [Green Version]
- Mamara, A.; Germenis, A.E.; Kompoti, M.; Palassopoulou, M.; Mandala, E.; Banti, A.; Giannakoulas, N.; Speletas, M. TACI expression and signaling in chronic lymphocytic leukemia. J. Immunol. Res. 2015, 2015, 478753. [Google Scholar] [CrossRef]
- Han, S.S.; Yang, S.H.; Choi, M.; Kim, H.R.; Kim, K.; Lee, S.; Moon, K.C.; Kim, J.Y.; Lee, H.; Lee, J.P.; et al. The Role of TNF Superfamily Member 13 in the Progression of IgA Nephropathy. J. Am. Soc. Nephrol. 2016, 27, 3430–3439. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Z.; Feng, S.Z.; Xu, R.C.; Li, Z.J.; Huang, F.X.; Yin, P.R.; Liu, W.T.; Wang, M.; Shi, D.C.; Zhou, Q.; et al. Association of TNFSF13 polymorphisms with IgA nephropathy in a Chinese Han population. J. Gene Med. 2017, 19, e2966. [Google Scholar] [CrossRef] [PubMed]
- Osman, W.; Okada, Y.; Kamatani, Y.; Kubo, M.; Matsuda, K.; Nakamura, Y. Association of common variants in TNFRSF13B, TNFSF13, and ANXA3 with serum levels of non-albumin protein and immunoglobulin isotypes in Japanese. PLoS ONE 2012, 7, e32683. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, A.; Tsuchiya, N.; Ohashi, J.; Murakami, Y.; Fukazawa, T.; Kusaoi, M.; Morimoto, S.; Matsuta, K.; Hashimoto, H.; Takasaki, Y.; et al. Role of APRIL (TNFSF13) polymorphisms in the susceptibility to systemic lupus erythematosus in Japanese. Rheumatology 2007, 46, 776–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.J.; Qi, Y.Y.; Hou, P.; Lv, J.C.; Shi, S.F.; Liu, L.J.; Zhao, N.; Zhang, H. Cumulative effects of variants identified by genome-wide association studies in IgA nephropathy. Sci. Rep. 2014, 4, 4904. [Google Scholar] [CrossRef] [Green Version]
- Consortium, E.P. An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57–74. [Google Scholar] [CrossRef]
- Davis, C.A.; Hitz, B.C.; Sloan, C.A.; Chan, E.T.; Davidson, J.M.; Gabdank, I.; Hilton, J.A.; Jain, K.; Baymuradov, U.K.; Narayanan, A.K.; et al. The Encyclopedia of DNA elements (ENCODE): Data portal update. Nucleic Acids Res. 2018, 46, D794–D801. [Google Scholar] [CrossRef] [Green Version]
- Consortium, G.T. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar]
- Klein, A.; Polliack, A.; Gafter-Gvili, A. Systemic lupus erythematosus and lymphoma: Incidence, pathogenesis and biology. Leuk. Res. 2018, 75, 45–49. [Google Scholar] [CrossRef]
- Ward, L.D.; Kellis, M. HaploReg: A resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012, 40, D930–D934. [Google Scholar] [CrossRef] [Green Version]
- Ward, L.D.; Kellis, M. HaploReg v4: Systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res. 2016, 44, D877–D881. [Google Scholar] [CrossRef]
- Vockley, C.M.; Barrera, A.; Reddy, T.E. Decoding the role of regulatory element polymorphisms in complex disease. Curr. Opin. Genet. Dev. 2017, 43, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Kofler, D.M.; Gawlik, B.B.; Elter, T.; Gianella-Borradori, A.; Wendtner, C.M.; Hallek, M. Phase 1b trial of atacicept, a recombinant protein binding BLyS and APRIL, in patients with chronic lymphocytic leukemia. Leukemia 2012, 26, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Samy, E.; Wax, S.; Huard, B.; Hess, H.; Schneider, P. Targeting BAFF and APRIL in systemic lupus erythematosus and other antibody-associated diseases. Int Rev. Immunol 2017, 36, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Kaegi, C.; Steiner, U.C.; Wuest, B.; Crowley, C.; Boyman, O. Systematic Review of Safety and Efficacy of Atacicept in Treating Immune-Mediated Disorders. Front. Immunol. 2020, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Merrill, J.T.; Wallace, D.J.; Wax, S.; Kao, A.; Fraser, P.A.; Chang, P.; Isenberg, D.; Investigators, A.I. Efficacy and Safety of Atacicept in Patients With Systemic Lupus Erythematosus: Results of a Twenty-Four-Week, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Arm, Phase IIb Study. Arthritis Rheumatol. 2018, 70, 266–276. [Google Scholar] [CrossRef] [Green Version]
- Lascano, V.; Guadagnoli, M.; Schot, J.G.; Luijks, D.M.; Guikema, J.E.; Cameron, K.; Hahne, M.; Pals, S.; Slinger, E.; Kipps, T.J.; et al. Chronic lymphocytic leukemia disease progression is accelerated by APRIL-TACI interaction in the TCL1 transgenic mouse model. Blood 2013, 122, 3960–3963. [Google Scholar] [CrossRef] [Green Version]
- Enjuanes, A.; Benavente, Y.; Bosch, F.; Martin-Guerrero, I.; Colomer, D.; Perez-Alvarez, S.; Reina, O.; Ardanaz, M.T.; Jares, P.; Garcia-Orad, A.; et al. Genetic variants in apoptosis and immunoregulation-related genes are associated with risk of chronic lymphocytic leukemia. Cancer Res. 2008, 68, 10178–10186. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Lam, K.P. Transmembrane Activator and CAML Interactor (TACI): Another Potential Target for Immunotherapy of Multiple Myeloma? Cancers 2020, 12, 1045. [Google Scholar] [CrossRef]
- Xu, Z.; Taylor, J.A. SNPinfo: Integrating GWAS and candidate gene information into functional SNP selection for genetic association studies. Nucleic Acids Res. 2009, 37, W600–W605. [Google Scholar] [CrossRef] [Green Version]
- Desmet, F.O.; Hamroun, D.; Lalande, M.; Collod-Beroud, G.; Claustres, M.; Beroud, C. Human Splicing Finder: An online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009, 37, e67. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.M.; Barber, G.P.; Casper, J.; Clawson, H.; Diekhans, M.; Gonzalez, J.N.; Hinrichs, A.S.; Lee, B.T.; Nassar, L.R.; Powell, C.C.; et al. UCSC Genome Browser enters 20th year. Nucleic Acids Res. 2020, 48, D756–D761. [Google Scholar] [CrossRef] [PubMed]
- Kent, W.J.; Sugnet, C.W.; Furey, T.S.; Roskin, K.M.; Pringle, T.H.; Zahler, A.M.; Haussler, D. The human genome browser at UCSC. Genome Res. 2002, 12, 996–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenbloom, K.R.; Sloan, C.A.; Malladi, V.S.; Dreszer, T.R.; Learned, K.; Kirkup, V.M.; Wong, M.C.; Maddren, M.; Fang, R.; Heitner, S.G.; et al. ENCODE data in the UCSC Genome Browser: Year 5 update. Nucleic Acids Res. 2013, 41, D56–D63. [Google Scholar] [CrossRef] [PubMed]
- Rousseeuw, P.J.; Croux, C. Alternatives to the Median Absolute Deviation. JASA 1993, 88, 1273–1283. [Google Scholar] [CrossRef]
- Rosnow, R.L.; Rosenthal, R.; Rubin, D.B. Contrasts and Correlations in Effect-Size Estimation. Psychol. Sci. 2000, 11, 446–453. [Google Scholar] [CrossRef]


| APRIL TNFSF13 polymorphisms | Patients (N = 439) | Controls (N = 477) | OR | CI95% | Patients vs. Controls | |||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | |||||
| rs3803800 a Asn96Ser | GG | 261 | 59.50 | 279 | 58.50 | 1 * | χ2df = 2 = 8.56 p = 0.014 | |
| GA | 142 | 32.30 | 179 | 37.50 | 0.85 | 0.64; 1.12 | ||
| AA | 36 | 8.20 | 19 | 4.00 | 2.00 | 1.13; 3.56 | ||
| HWE | p = 0.013 | p = 0.154 | ||||||
| f = 0.12 | f = −0.07 | |||||||
| CI95% = 0.02;0.22 | CI95% = −0.15; 0.02 | |||||||
| TACI TNFRSF13B polymorphisms | Patients (N = 439) | Controls (N = 477) | OR | CI95% | Patients vs. Controls | |||
| N | % | N | % | |||||
| rs4985726 intron 1 | CC | 353 | 80.40 | 353 | 74.00 | 1 * | χ2df = 2 = 6.30 p = 0.042 | |
| CG | 78 | 17.80 | 117 | 24.50 | 0.67 | 0.48; 0.92 | ||
| GG | 8 | 1.80 | 7 | 1.50 | 1.13 | 0.42; 3.06 | ||
| HWE | p = 0.135 | p = 0.56 | ||||||
| f = 0.07 | f = −0.03 | |||||||
| CI95% = −0.04;0.18 | CI95% = −0.11;0.048 | |||||||
| TACI MFI | Genotype | F-test | p-value | LSD | ||
|---|---|---|---|---|---|---|
| rs11078355 | AA | AG | GG | |||
| average | 34.72 | 43.40 | 41.90 | 4.102 | 0.0185 | AA (AG.GG) |
| n | 58 | 65 | 22 | |||
| CD19+TACI+ % | ||||||
| rs11078355 | AA | AG | GG | |||
| average | 12.13 | 7.12 | 15.35 | 3.41 | 0.0357 | AG (AA.GG) |
| n | 59 | 65 | 22 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasek, M.; Bojarska-Junak, A.; Sobczyński, M.; Wagner, M.; Chocholska, S.; Roliński, J.; Wołowiec, D.; Karabon, L. Association of Common Variants of TNFSF13 and TNFRSF13B Genes with CLL Risk and Clinical Picture, as Well as Expression of Their Products—APRIL and TACI Molecules. Cancers 2020, 12, 2873. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12102873
Jasek M, Bojarska-Junak A, Sobczyński M, Wagner M, Chocholska S, Roliński J, Wołowiec D, Karabon L. Association of Common Variants of TNFSF13 and TNFRSF13B Genes with CLL Risk and Clinical Picture, as Well as Expression of Their Products—APRIL and TACI Molecules. Cancers. 2020; 12(10):2873. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12102873
Chicago/Turabian StyleJasek, Monika, Agnieszka Bojarska-Junak, Maciej Sobczyński, Marta Wagner, Sylwia Chocholska, Jacek Roliński, Dariusz Wołowiec, and Lidia Karabon. 2020. "Association of Common Variants of TNFSF13 and TNFRSF13B Genes with CLL Risk and Clinical Picture, as Well as Expression of Their Products—APRIL and TACI Molecules" Cancers 12, no. 10: 2873. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12102873