Tumour Dissemination in Multiple Myeloma Disease Progression and Relapse: A Potential Therapeutic Target in High-Risk Myeloma
Abstract
:Simple Summary
Abstract
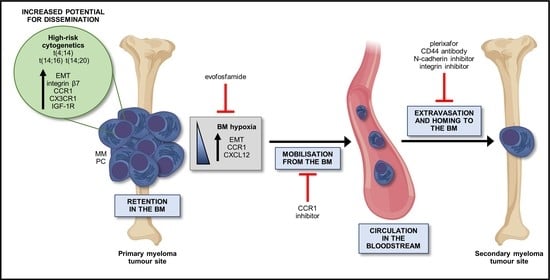
1. The Role of Dissemination in the Progression of Multiple Myeloma
2. The Process of Dissemination in MM
2.1. Retention within the BM Stromal Niche
2.2. Release from the BM Niche
2.3. Microenvironmental Control of Release from the BM Niche
2.4. Intravasation
2.5. Extravasation and Homing to the BM
2.6. Establishment and Colonisation of MM PCs in a New BM Niche
3. Intrinsic MM Characteristics that May Facilitate Dissemination
3.1. t(14;16) and t(14;20)
3.2. t(4;14)
3.3. Subclonal Heterogeneity and Dissemination
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.J.; Allen, C.; Barac, A.; Basaleem, H.; Bensenor, I.; Curado, M.P.; Foreman, K.; Gupta, R.; Harvey, J.; Hosgood, H.D.; et al. Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA Oncol. 2018, 4, 1221–1227. [Google Scholar] [CrossRef] [Green Version]
- Noll, J.E.; Williams, S.A.; Purton, L.E.; Zannettino, A.C.W. Tug of war in the haematopoietic stem cell niche: Do myeloma plasma cells compete for the HSC niche? Blood Cancer J. 2012, 2, e91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillengass, J.; Fechtner, K.; Weber, M.A.; Bauerle, T.; Ayyaz, S.; Heiss, C.; Hielscher, T.; Moehler, T.M.; Egerer, G.; Neben, K.; et al. Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J. Clin. Oncol. 2010, 28, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, M.V.; Sexton, R.; Waheed, S.; Usmani, S.; Papanikolaou, X.; Nair, B.; Petty, N.; Shaughnessy, J.D., Jr.; Hoering, A.; Crowley, J.; et al. Clinical, genomic, and imaging predictors of myeloma progression from asymptomatic monoclonal gammopathies (SWOG S0120). Blood 2014, 123, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Kastritis, E.; Moulopoulos, L.A.; Terpos, E.; Koutoulidis, V.; Dimopoulos, M.A. The prognostic importance of the presence of more than one focal lesion in spine MRI of patients with asymptomatic (smoldering) multiple myeloma. Leukemia 2014, 28, 2402–2403. [Google Scholar] [CrossRef]
- Walker, R.; Barlogie, B.; Haessler, J.; Tricot, G.; Anaissie, E.; Shaughnessy, J.D., Jr.; Epstein, J.; van Hemert, R.; Erdem, E.; Hoering, A.; et al. Magnetic resonance imaging in multiple myeloma: Diagnostic and clinical implications. J. Clin. Oncol. 2007, 25, 1121–1128. [Google Scholar] [CrossRef] [Green Version]
- Mai, E.K.; Hielscher, T.; Kloth, J.K.; Merz, M.; Shah, S.; Raab, M.S.; Hillengass, M.; Wagner, B.; Jauch, A.; Hose, D.; et al. A magnetic resonance imaging-based prognostic scoring system to predict outcome in transplant-eligible patients with multiple myeloma. Haematologica 2015, 100, 818–825. [Google Scholar] [CrossRef] [Green Version]
- Nowakowski, G.S.; Witzig, T.E.; Dingli, D.; Tracz, M.J.; Gertz, M.A.; Lacy, M.Q.; Lust, J.A.; Dispenzieri, A.; Greipp, P.R.; Kyle, R.A.; et al. Circulating plasma cells detected by flow cytometry as a predictor of survival in 302 patients with newly diagnosed multiple myeloma. Blood 2005, 106, 2276–2279. [Google Scholar] [CrossRef] [Green Version]
- Granell, M.; Calvo, X.; Garcia-Guiñón, A.; Escoda, L.; Abella, E.; Martínez, C.M.; Teixidó, M.; Gimenez, M.T.; Senín, A.; Sanz, P.; et al. Prognostic impact of circulating plasma cells in patients with multiple myeloma: Implications for plasma cell leukemia definition. Haematologica 2017, 102, 1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witzig, T.E.; Gertz, M.A.; Lust, J.A.; Kyle, R.A.; O’Fallon, W.M.; Greipp, P.R. Peripheral blood monoclonal plasma cells as a predictor of survival in patients with multiple myeloma. Blood 1996, 88, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Dingli, D.; Nowakowski, G.S.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Rajkumar, S.V.; Greipp, P.R.; Litzow, M.R.; Gastineau, D.A.; Witzig, T.E.; et al. Flow cytometric detection of circulating myeloma cells before transplantation in patients with multiple myeloma: A simple risk stratification system. Blood 2006, 107, 3384–3388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonsalves, W.I.; Rajkumar, S.V.; Gupta, V.; Morice, W.G.; Timm, M.M.; Singh, P.P.; Dispenzieri, A.; Buadi, F.K.; Lacy, M.Q.; Kapoor, P.; et al. Quantification of clonal circulating plasma cells in newly diagnosed multiple myeloma: Implications for redefining high-risk myeloma. Leukemia 2014, 28, 2060–2065. [Google Scholar] [CrossRef] [Green Version]
- Fernandez de Larrea, C.; Kyle, R.A.; Durie, B.G.; Ludwig, H.; Usmani, S.; Vesole, D.H.; Hajek, R.; San Miguel, J.F.; Sezer, O.; Sonneveld, P.; et al. Plasma cell leukemia: Consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia 2013, 27, 780–791. [Google Scholar] [CrossRef] [Green Version]
- Jagosky, M.H.; Usmani, S.Z. Extramedullary Disease in Multiple Myeloma. Curr. Hematolog. Malig. Rep. 2020, 15, 62–71. [Google Scholar] [CrossRef]
- Dutta, A.K.; Fink, J.L.; Grady, J.P.; Morgan, G.J.; Mullighan, C.G.; To, L.B.; Hewett, D.R.; Zannettino, A.C.W. Subclonal evolution in disease progression from MGUS/SMM to multiple myeloma is characterised by clonal stability. Leukemia 2018, 33, 457–468. [Google Scholar] [CrossRef]
- Walker, B.A.; Wardell, C.P.; Melchor, L.; Brioli, A.; Johnson, D.C.; Kaiser, M.F.; Mirabella, F.; Lopez-Corral, L.; Humphray, S.; Murray, L.; et al. Intraclonal heterogeneity is a critical early event in the development of myeloma and precedes the development of clinical symptoms. Leukemia 2013, 28, 384. [Google Scholar] [CrossRef] [Green Version]
- Bustoros, M.; Sklavenitis-Pistofidis, R.; Park, J.; Redd, R.; Zhitomirsky, B.; Dunford, A.J.; Salem, K.; Tai, Y.T.; Anand, S.; Mouhieddine, T.H.; et al. Genomic Profiling of Smoldering Multiple Myeloma Identifies Patients at a High Risk of Disease Progression. J. Clin. Oncol. 2020, 38, 2380–2389. [Google Scholar] [CrossRef]
- Bianchi, G.; Kyle, R.A.; Larson, D.R.; Witzig, T.E.; Kumar, S.; Dispenzieri, A.; Morice, W.G.; Rajkumar, S.V. High levels of peripheral blood circulating plasma cells as a specific risk factor for progression of smoldering multiple myeloma. Leukemia 2013, 27, 680–685. [Google Scholar] [CrossRef]
- Gonsalves, W.I.; Rajkumar, S.V.; Dispenzieri, A.; Dingli, D.; Timm, M.M.; Morice, W.G.; Lacy, M.Q.; Buadi, F.K.; Go, R.S.; Leung, N.; et al. Quantification of circulating clonal plasma cells via multiparametric flow cytometry identifies patients with smoldering multiple myeloma at high risk of progression. Leukemia 2017, 31, 130–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz-Rodríguez, F.; Ruiz-Velasco, N.; Pascual-Salcedo, D.; Teixidó, J. Characterization of VLA-4-dependent myeloma cell adhesion to fibronectin and VCAM-1. Br. J. Haematol. 1999, 107, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, H.; Tagawa, S.; Hattori, H.; Inoue, R.; Katagiri, S.; Kitani, T. Laminin and fibronectin promote the chemotaxis of human malignant plasma cell lines. Blood 1995, 86, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, T.; Chauhan, D.; Schlossman, R.; Richardson, P.; Anderson, K.C. The role of tumor necrosis factor alpha in the pathophysiology of human multiple myeloma: Therapeutic applications. Oncogene 2001, 20, 4519–4527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Driel, M.; Günthert, U.; van Kessel, A.C.; Joling, P.; Stauder, R.; Lokhorst, H.M.; Bloem, A.C. CD44 variant isoforms are involved in plasma cell adhesion to bone marrow stromal cells. Leukemia 2002, 16, 135. [Google Scholar] [CrossRef] [Green Version]
- Ridley, R.C.; Xiao, H.; Hata, H.; Woodliff, J.; Epstein, J.; Sanderson, R.D. Expression of syndecan regulates human myeloma plasma cell adhesion to type I collagen. Blood 1993, 81, 767–774. [Google Scholar] [CrossRef] [Green Version]
- Sanz-Rodriguez, F.; Hidalgo, A.; Teixido, J. Chemokine stromal cell-derived factor-1alpha modulates VLA-4 integrin-mediated multiple myeloma cell adhesion to CS-1/fibronectin and VCAM-1. Blood 2001, 97, 346–351. [Google Scholar] [CrossRef] [Green Version]
- Alsayed, Y.; Ngo, H.; Runnels, J.; Leleu, X.; Singha, U.K.; Pitsillides, C.M.; Spencer, J.A.; Kimlinger, T.; Ghobrial, J.M.; Jia, X.; et al. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood 2007, 109, 2708–2717. [Google Scholar] [CrossRef]
- Ponomaryov, T.; Peled, A.; Petit, I.; Taichman, R.S.; Habler, L.; Sandbank, J.; Arenzana-Seisdedos, F.; Magerus, A.; Caruz, A.; Fujii, N.; et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J. Clin. Investig. 2000, 106, 1331–1339. [Google Scholar] [CrossRef] [Green Version]
- Tokoyoda, K.; Egawa, T.; Sugiyama, T.; Choi, B.I.; Nagasawa, T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity 2004, 20, 707–718. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, T.; Hieshima, K.; Izawa, D.; Tatsumi, Y.; Kanamaru, A.; Yoshie, O. Cutting edge: Profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues. J. Immunol. 2003, 170, 1136–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honczarenko, M.; Le, Y.; Swierkowski, M.; Ghiran, I.; Glodek, A.M.; Silberstein, L.E. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 2006, 24, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, A.; Ria, R.; Di Pietro, G.; Cirulli, T.; Surico, G.; Pennisi, A.; Morabito, F.; Ribatti, D.; Vacca, A. Bone marrow endothelial cells in multiple myeloma secrete CXC-chemokines that mediate interactions with plasma cells. Br. J. Haematol. 2005, 129, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Vande Broek, I.; Leleu, X.; Schots, R.; Facon, T.; Vanderkerken, K.; Van Camp, B.; Van Riet, I. Clinical significance of chemokine receptor (CCR1, CCR2 and CXCR4) expression in human myeloma cells: The association with disease activity and survival. Haematologica 2006, 91, 200–206. [Google Scholar] [PubMed]
- Vandyke, K.; Zeissig, M.N.; Hewett, D.R.; Martin, S.K.; Mrozik, K.M.; Cheong, C.M.; Diamond, P.; To, L.B.; Gronthos, S.; Peet, D.J.; et al. HIF-2α promotes dissemination of plasma cells in multiple myeloma by regulating CXCL12/CXCR4 and CCR1. Cancer Res. 2017, 77, 5452–5463. [Google Scholar] [CrossRef] [Green Version]
- Azab, A.K.; Runnels, J.M.; Pitsillides, C.; Moreau, A.-S.; Azab, F.; Leleu, X.; Jia, X.; Wright, R.; Ospina, B.; Carlson, A.L.; et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood 2009, 113, 4341–4351. [Google Scholar] [CrossRef] [Green Version]
- Urashima, M.; Chauhan, D.; Uchiyama, H.; Freeman, G.J.; Anderson, K.C. CD40 ligand triggered interleukin-6 secretion in multiple myeloma. Blood 1995, 85, 1903–1912. [Google Scholar] [CrossRef]
- Tai, Y.T.; Podar, K.; Mitsiades, N.; Lin, B.; Mitsiades, C.; Gupta, D.; Akiyama, M.; Catley, L.; Hideshima, T.; Munshi, N.C.; et al. CD40 induces human multiple myeloma cell migration via phosphatidylinositol 3-kinase/AKT/NF-kappa B signaling. Blood 2003, 101, 2762–2769. [Google Scholar] [CrossRef]
- Moreaux, J.; Cremer, F.W.; Reme, T.; Raab, M.; Mahtouk, K.; Kaukel, P.; Pantesco, V.; De Vos, J.; Jourdan, E.; Jauch, A.; et al. The level of TACI gene expression in myeloma cells is associated with a signature of microenvironment dependence versus a plasmablastic signature. Blood 2005, 106, 1021–1030. [Google Scholar] [CrossRef] [Green Version]
- Tai, Y.T.; Li, X.F.; Breitkreutz, I.; Song, W.; Neri, P.; Catley, L.; Podar, K.; Hideshima, T.; Chauhan, D.; Raje, N.; et al. Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res. 2006, 66, 6675–6682. [Google Scholar] [CrossRef] [Green Version]
- Paiva, B.; Paino, T.; Sayagues, J.M.; Garayoa, M.; San-Segundo, L.; Martin, M.; Mota, I.; Sanchez, M.L.; Barcena, P.; Aires-Mejia, I.; et al. Detailed characterization of multiple myeloma circulating tumor cells shows unique phenotypic, cytogenetic, functional, and circadian distribution profile. Blood 2013, 122, 3591–3598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paiva, B.; Perez-Andres, M.; Vidriales, M.B.; Almeida, J.; de las Heras, N.; Mateos, M.V.; Lopez-Corral, L.; Gutierrez, N.C.; Blanco, J.; Oriol, A.; et al. Competition between clonal plasma cells and normal cells for potentially overlapping bone marrow niches is associated with a progressively altered cellular distribution in MGUS vs myeloma. Leukemia 2011, 25, 697–706. [Google Scholar] [CrossRef] [Green Version]
- Luque, R.; Garcia-Trujillo, J.A.; Camara, C.; Moreno, A.; Eiras, P.; Roy, G.; Villar, L.M.; Lombardia, M.; Brieva, J.A.; Bootello, A.; et al. CD106 and activated-CD29 are expressed on myelomatous bone marrow plasma cells and their downregulation is associated with tumour progression. Br. J. Haematol. 2002, 119, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Akhmetzyanova, I.; McCarron, M.J.; Parekh, S.; Chesi, M.; Bergsagel, P.L.; Fooksman, D.R. Dynamic CD138 surface expression regulates switch between myeloma growth and dissemination. Leukemia 2020, 34, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Macleod, V.; Bendre, M.; Huang, Y.; Theus, A.M.; Miao, H.Q.; Kussie, P.; Yaccoby, S.; Epstein, J.; Suva, L.J.; et al. Heparanase promotes the spontaneous metastasis of myeloma cells to bone. Blood 2005, 105, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Andrés, M.; Almeida, J.; Martín-Ayuso, M.; Moro, M.J.; Martín-Nuñez, G.; Galende, J.; Borrego, D.; Rodríguez, M.J.; Ortega, F.; Hernandez, J.; et al. Clonal plasma cells from monoclonal gammopathy of undetermined significance, multiple myeloma and plasma cell leukemia show different expression profiles of molecules involved in the interaction with the immunological bone marrow microenvironment. Leukemia 2005, 19, 449. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.K.; Diamond, P.; Gronthos, S.; Peet, D.J.; Zannettino, A.C. The emerging role of hypoxia, HIF-1 and HIF-2 in multiple myeloma. Leukemia 2011, 25, 1533–1542. [Google Scholar] [CrossRef] [Green Version]
- Azab, A.K.; Hu, J.; Quang, P.; Azab, F.; Pitsillides, C.M.; Awwad, R.; Thompson, B.; Maiso, P.; Sun, J.D.; Hart, C.P.; et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood 2012, 119, 5782–5794. [Google Scholar] [CrossRef] [Green Version]
- Storti, P.; Bolzoni, M.; Donofrio, G.; Airoldi, I.; Guasco, D.; Toscani, D.; Martella, E.; Lazzaretti, M.; Mancini, C.; Agnelli, L.; et al. Hypoxia-inducible factor (HIF)-1alpha suppression in myeloma cells blocks tumoral growth in vivo inhibiting angiogenesis and bone destruction. Leukemia 2013, 27, 1697–1706. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Handisides, D.R.; Van Valckenborgh, E.; De Raeve, H.; Menu, E.; Vande Broek, I.; Liu, Q.; Sun, J.D.; Van Camp, B.; Hart, C.P.; et al. Targeting the multiple myeloma hypoxic niche with TH-302, a hypoxia-activated prodrug. Blood 2010, 116, 1524–1527. [Google Scholar] [CrossRef] [Green Version]
- Garces, J.J.; Simicek, M.; Vicari, M.; Brozova, L.; Burgos, L.; Bezdekova, R.; Alignani, D.; Calasanz, M.J.; Growkova, K.; Goicoechea, I.; et al. Transcriptional profiling of circulating tumor cells in multiple myeloma: A new model to understand disease dissemination. Leukemia 2020, 34, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Kikukawa, Y.; Fujiwara, S.; Wada, N.; Okuno, Y.; Mitsuya, H.; Hata, H. Hypoxia reduces CD138 expression and induces an immature and stem cell-like transcriptional program in myeloma cells. Int. J. Oncol. 2013, 43, 1809–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheong, C.M.; Mrozik, K.M.; Hewett, D.R.; Bell, E.; Panagopoulos, V.; Noll, J.E.; Licht, J.D.; Gronthos, S.; Zannettino, A.C.W.; Vandyke, K. Twist-1 is upregulated by NSD2 and contributes to tumour dissemination and an epithelial-mesenchymal transition-like gene expression signature in t(4;14)-positive multiple myeloma. Cancer Lett. 2020, 475, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.K.; Diamond, P.; Williams, S.A.; To, L.B.; Peet, D.J.; Fujii, N.; Gronthos, S.; Harris, A.L.; Zannettino, A.C.W. Hypoxia-inducible factor-2 is a novel regulator of aberrant CXCL12 expression in multiple myeloma plasma cells. Haematologica 2010, 95, 776–784. [Google Scholar] [CrossRef] [Green Version]
- Zeissig, M.N.; Hewett, D.R.; Panagopoulos, V.; Mrozik, K.M.; To, L.B.; Croucher, P.I.; Zannettino, A.C.W.; Vandyke, K. Expression of the chemokine receptor CCR1 promotes the dissemination of multiple myeloma plasma cells in vivo. Haematologica 2020. [Google Scholar] [CrossRef]
- Barille, S.; Akhoundi, C.; Collette, M.; Mellerin, M.P.; Rapp, M.J.; Harousseau, J.L.; Bataille, R.; Amiot, M. Metalloproteinases in multiple myeloma: Production of matrix metalloproteinase-9 (MMP-9), activation of proMMP-2, and induction of MMP-1 by myeloma cells. Blood 1997, 90, 1649–1655. [Google Scholar] [CrossRef]
- Asosingh, K.; Menu, E.; Van Valckenborgh, E.; Vande Broek, I.; Van Riet, I.; Van Camp, B.; Vanderkerken, K. Mechanisms involved in the differential bone marrow homing of CD45 subsets in 5T murine models of myeloma. Clin. Exp. Metastasis 2002, 19, 583. [Google Scholar] [CrossRef]
- Vanderkerken, K.; Vande Broek, I.; Eizirik, D.L.; Van Valckenborgh, E.; Asosingh, K.; Van Riet, I.; Van Camp, B. Monocyte chemoattractant protein-1 (MCP-1), secreted by bone marrow endothelial cells, induces chemoattraction of 5T multiple myeloma cells. Clin. Exp. Metastasis 2002, 19, 87–90. [Google Scholar] [CrossRef]
- Johrer, K.; Janke, K.; Krugmann, J.; Fiegl, M.; Greil, R. Transendothelial migration of myeloma cells is increased by tumor necrosis factor (TNF)-alpha via TNF receptor 2 and autocrine up-regulation of MCP-1. Clin. Cancer Res. 2004, 10, 1901–1910. [Google Scholar] [CrossRef] [Green Version]
- Kunkel, E.J.; Butcher, E.C. Plasma-cell homing. Nat. Rev. Immunol. 2003, 3, 822–829. [Google Scholar] [CrossRef]
- Okada, T.; Hawley, R.G.; Kodaka, M.; Okuno, H. Significance of VLA-4-VCAM-1 interaction and CD44 for transendothelial invasion in a bone marrow metastatic myeloma model. Clin. Exp. Metastasis 1999, 17, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Yago, T.; Shao, B.; Miner, J.J.; Yao, L.; Klopocki, A.G.; Maeda, K.; Coggeshall, K.M.; McEver, R.P. E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin αLβ2-mediated slow leukocyte rolling. Blood 2010, 116, 485–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asosingh, K.; Günthert, U.; De Raeve, H.; Van Riet, I.; Van Camp, B.; Vanderkerken, K. A unique pathway in the homing of murine multiple myeloma cells: CD44v10 mediates binding to bone marrow endothelium. Cancer Res. 2001, 61, 2862. [Google Scholar] [PubMed]
- Azab, A.K.; Quang, P.; Azab, F.; Pitsillides, C.; Thompson, B.; Chonghaile, T.; Patton, J.T.; Maiso, P.; Monrose, V.; Sacco, A.; et al. P-selectin glycoprotein ligand regulates the interaction of multiple myeloma cells with the bone marrow microenvironment. Blood 2012, 119, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Mrozik, K.M.; Cheong, C.M.; Hewett, D.; Chow, A.W.; Blaschuk, O.W.; Zannettino, A.C.; Vandyke, K. Therapeutic targeting of N-cadherin is an effective treatment for multiple myeloma. Br. J. Haematol. 2015, 171, 387–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groen, R.W.J.; de Rooij, M.F.M.; Kocemba, K.A.; Reijmers, R.M.; de Haan-Kramer, A.; Overdijk, M.B.; Aalders, L.; Rozemuller, H.; Martens, A.C.M.; Bergsagel, P.L.; et al. N-cadherin-mediated interaction with multiple myeloma cells inhibits osteoblast differentiation. Haematologica 2011, 96, 1653–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, K.; Kobayashi, M.; Wang, J.; Shinobu, N.; Yoshida, H.; Hamada, J.; Shindo, M.; Higashino, F.; Tanaka, J.; Asaka, M.; et al. Selective secretion of chemoattractants for haemopoietic progenitor cells by bone marrow endothelial cells: A possible role in homing of haemopoietic progenitor cells to bone marrow. Br. J. Haematol. 1999, 106, 905–911. [Google Scholar] [CrossRef]
- Sipkins, D.A.; Wei, X.; Wu, J.W.; Runnels, J.M.; Cote, D.; Means, T.K.; Luster, A.D.; Scadden, D.T.; Lin, C.P. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 2005, 435, 969–973. [Google Scholar] [CrossRef]
- Vanderkerken, K.; Greef, C.D.; Asosingh, K.; Arteta, B.; Veerman, M.D.; Broek, I.V.; Riet, I.V.; Kobayashi, M.; Smedsrod, B.; Camp, B.V. Selective initial in vivo homing pattern of 5T2 multiple myeloma cells in the C57BL/KalwRij mouse. Br. J. Cancer 2000, 82, 953–959. [Google Scholar] [CrossRef] [Green Version]
- Parmo-Cabañas, M.; Bartolomé, R.A.; Wright, N.; Hidalgo, A.; Drager, A.M.; Teixidó, J. Integrin α4β1 involvement in stromal cell-derived factor-1α-promoted myeloma cell transendothelial migration and adhesion: Role of cAMP and the actin cytoskeleton in adhesion. Exp. Cell Res. 2004, 294, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Moreno, M.; Leiva, M.; Aguilera-Montilla, N.; Sevilla-Movilla, S.; Isern de Val, S.; Arellano-Sánchez, N.; Gutiérrez, N.C.; Maldonado, R.; Martínez-López, J.; Buño, I.; et al. In vivo adhesion of malignant B cells to bone marrow microvasculature is regulated by α4β1 cytoplasmic-binding proteins. Leukemia 2016, 30, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Runnels, J.M.; Carlson, A.L.; Pitsillides, C.; Thompson, B.; Wu, J.; Spencer, J.A.; Kohler, J.M.; Azab, A.; Moreau, A.S.; Rodig, S.J.; et al. Optical techniques for tracking multiple myeloma engraftment, growth, and response to therapy. J. Biomed. Opt. 2011, 16, 011006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vande Broek, I.; Asosingh, K.; Allegaert, V.; Leleu, X.; Facon, T.; Vanderkerken, K.; Van Camp, B.; Van Riet, I. Bone marrow endothelial cells increase the invasiveness of human multiple myeloma cells through upregulation of MMP-9: Evidence for a role of hepatocyte growth factor. Leukemia 2004, 18, 976–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menu, E.; Asosingh, K.; Indraccolo, S.; De Raeve, H.; Van Riet, I.; Van Valckenborgh, E.; Van de Broek, I.; Fujii, N.; Tamamura, H.; Van Camp, B.; et al. The involvement of stromal derived factor 1alpha in homing and progression of multiple myeloma in the 5TMM model. Haematologica 2006, 91, 605–612. [Google Scholar] [PubMed]
- Vande Broek, I.; Vanderkerken, K.; Van Camp, B.; Van Riet, I. Extravasation and homing mechanisms in multiple myeloma. Clin. Exp. Metastasis 2008, 25, 325–334. [Google Scholar] [CrossRef]
- Moller, C.; Stromberg, T.; Juremalm, M.; Nilsson, K.; Nilsson, G. Expression and function of chemokine receptors in human multiple myeloma. Leukemia 2003, 17, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Roccaro, A.M.; Mishima, Y.; Sacco, A.; Moschetta, M.; Tai, Y.T.; Shi, J.; Zhang, Y.; Reagan, M.R.; Huynh, D.; Kawano, Y.; et al. CXCR4 Regulates Extra-Medullary Myeloma through Epithelial-Mesenchymal-Transition-like Transcriptional Activation. Cell Rep. 2015, 12, 622–635. [Google Scholar] [CrossRef] [Green Version]
- Roccaro, A.M.; Sacco, A.; Purschke, W.G.; Moschetta, M.; Buchner, K.; Maasch, C.; Zboralski, D.; Zollner, S.; Vonhoff, S.; Mishima, Y.; et al. SDF-1 inhibition targets the bone marrow niche for cancer therapy. Cell Rep. 2014, 9, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Takai, K.; Hara, J.; Matsumoto, K.; Hosoi, G.; Osugi, Y.; Tawa, A.; Okada, S.; Nakamura, T. Hepatocyte growth factor is constitutively produced by human bone marrow stromal cells and indirectly promotes hematopoiesis. Blood 1997, 89, 1560–1565. [Google Scholar] [CrossRef] [Green Version]
- Zdzisinska, B.; Bojarska-Junak, A.; Dmoszynska, A.; Kandefer-Szerszen, M. Abnormal cytokine production by bone marrow stromal cells of multiple myeloma patients in response to RPMI8226 myeloma cells. Arch. Immunol. Ther. Exp. 2008, 56, 207–221. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ro, T.B.; Holien, T.; Fagerli, U.M.; Hov, H.; Misund, K.; Waage, A.; Sundan, A.; Holt, R.U.; Borset, M. HGF and IGF-1 synergize with SDF-1alpha in promoting migration of myeloma cells by cooperative activation of p21-activated kinase. Exp. Hematol. 2013, 41, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hendrix, A.; Hernot, S.; Lemaire, M.; De Bruyne, E.; Van Valckenborgh, E.; Lahoutte, T.; De Wever, O.; Vanderkerken, K.; Menu, E. Bone marrow stromal cell-derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood 2014, 124, 555–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asosingh, K.; Gunthert, U.; Bakkus, M.H.; De Raeve, H.; Goes, E.; Van Riet, I.; Van Camp, B.; Vanderkerken, K. In vivo induction of insulin-like growth factor-I receptor and CD44v6 confers homing and adhesion to murine multiple myeloma cells. Cancer Res. 2000, 60, 3096–3104. [Google Scholar]
- Opperman, K.S.; Vandyke, K.; Clark, K.C.; Coulter, E.A.; Hewett, D.R.; Mrozik, K.M.; Schwarz, N.; Evdokiou, A.; Croucher, P.I.; Psaltis, P.J.; et al. Clodronate-liposome mediated macrophage depletion abrogates multiple myeloma tumor establishment in vivo. Neoplasia 2019, 21, 777–787. [Google Scholar] [CrossRef]
- Davatelis, G.; Tekamp-Olson, P.; Wolpe, S.D.; Hermsen, K.; Luedke, C.; Gallegos, C.; Coit, D.; Merryweather, J.; Cerami, A. Cloning and characterization of a cDNA for murine macrophage inflammatory protein (MIP), a novel monokine with inflammatory and chemokinetic properties. J. Exp. Med. 1988, 167, 1939–1944. [Google Scholar] [CrossRef] [Green Version]
- Moreaux, J.; Hose, D.; Kassambara, A.; Reme, T.; Moine, P.; Requirand, G.; Goldschmidt, H.; Klein, B. Osteoclast-gene expression profiling reveals osteoclast-derived CCR2 chemokines promoting myeloma cell migration. Blood 2011, 117, 1280–1290. [Google Scholar] [CrossRef] [Green Version]
- Lentzsch, S.; Gries, M.; Janz, M.; Bargou, R.; Dorken, B.; Mapara, M.Y. Macrophage inflammatory protein 1-alpha (MIP-1 alpha ) triggers migration and signaling cascades mediating survival and proliferation in multiple myeloma (MM) cells. Blood 2003, 101, 3568–3573. [Google Scholar] [CrossRef] [Green Version]
- Vallet, S.; Raje, N.; Ishitsuka, K.; Hideshima, T.; Podar, K.; Chhetri, S.; Pozzi, S.; Breitkreutz, I.; Kiziltepe, T.; Yasui, H.; et al. MLN3897, a novel CCR1 inhibitor, impairs osteoclastogenesis and inhibits the interaction of multiple myeloma cells and osteoclasts. Blood 2007, 110, 3744–3752. [Google Scholar] [CrossRef] [Green Version]
- Vande Broek, I.; Asosingh, K.; Vanderkerken, K.; Straetmans, N.; Van Camp, B.; Van Riet, I. Chemokine receptor CCR2 is expressed by human multiple myeloma cells and mediates migration to bone marrow stromal cell-produced monocyte chemotactic proteins MCP-1, -2 and -3. Br. J. Cancer 2003, 88, 855–862. [Google Scholar] [CrossRef] [Green Version]
- Pellegrino, A.; Antonaci, F.; Russo, F.; Merchionne, F.; Ribatti, D.; Vacca, A.; Dammacco, F. CXCR3-binding chemokines in multiple myeloma. Cancer Lett. 2004, 207, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, H.N.; Kim, K.O.; Jin, W.J.; Lee, S.; Kim, H.H.; Ha, H.; Lee, Z.H. CXCL10 promotes osteolytic bone metastasis by enhancing cancer outgrowth and osteoclastogenesis. Cancer Res. 2012, 72, 3175–3186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caers, J.; Deleu, S.; Belaid, Z.; De Raeve, H.; Van Valckenborgh, E.; De Bruyne, E.; Defresne, M.P.; Van Riet, I.; Van Camp, B.; Vanderkerken, K. Neighboring adipocytes participate in the bone marrow microenvironment of multiple myeloma cells. Leukemia 2007, 21, 1580–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trotter, T.N.; Gibson, J.T.; Sherpa, T.L.; Gowda, P.S.; Peker, D.; Yang, Y. Adipocyte-Lineage Cells Support Growth and Dissemination of Multiple Myeloma in Bone. Am. J. Pathol. 2016, 186, 3054–3063. [Google Scholar] [CrossRef] [Green Version]
- Morris, E.V.; Suchacki, K.J.; Hocking, J.; Cartwright, R.; Sowman, A.; Gamez, B.; Lea, R.; Drake, M.T.; Cawthorn, W.P.; Edwards, C.M. Myeloma cells down-regulate adiponectin in bone marrow adipocytes via TNF-Alpha. J. Bone Miner. Res. 2020, 35, 942–955. [Google Scholar] [CrossRef] [Green Version]
- Geng, S.; Wang, J.; Zhang, X.; Zhang, J.J.; Wu, F.; Pang, Y.; Zhong, Y.; Wang, J.; Wang, W.; Lyu, X.; et al. Single-cell RNA sequencing reveals chemokine self-feeding of myeloma cells promotes extramedullary metastasis. FEBS Lett. 2020, 594, 452–465. [Google Scholar] [CrossRef] [Green Version]
- Uneda, S.; Hata, H.; Matsuno, F.; Harada, N.; Mitsuya, Y.; Kawano, F.; Mitsuya, H. Macrophage inflammatory protein-1 alpha is produced by human multiple myeloma (MM) cells and its expression correlates with bone lesions in patients with MM. Br. J. Haematol. 2003, 120, 53–55. [Google Scholar] [CrossRef]
- Roussou, M.; Tasidou, A.; Dimopoulos, M.A.; Kastritis, E.; Migkou, M.; Christoulas, D.; Gavriatopoulou, M.; Zagouri, F.; Matsouka, C.; Anagnostou, D.; et al. Increased expression of macrophage inflammatory protein-1alpha on trephine biopsies correlates with extensive bone disease, increased angiogenesis and advanced stage in newly diagnosed patients with multiple myeloma. Leukemia 2009, 23, 2177–2181. [Google Scholar] [CrossRef] [Green Version]
- Bataille, R.; Robillard, N.; Avet-Loiseau, H.; Harousseau, J.L.; Moreau, P. CD221 (IGF-1R) is aberrantly expressed in multiple myeloma, in relation to disease severity. Haematologica 2005, 90, 706–707. [Google Scholar]
- Sprynski, A.C.; Hose, D.; Caillot, L.; Reme, T.; Shaughnessy, J.D., Jr.; Barlogie, B.; Seckinger, A.; Moreaux, J.; Hundemer, M.; Jourdan, M.; et al. The role of IGF-1 as a major growth factor for myeloma cell lines and the prognostic relevance of the expression of its receptor. Blood 2009, 113, 4614–4626. [Google Scholar] [CrossRef]
- Wynes, M.W.; Riches, D.W. Induction of macrophage insulin-like growth factor-I expression by the Th2 cytokines IL-4 and IL-13. J. Immunol. 2003, 171, 3550–3559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borset, M.; Hjorth-Hansen, H.; Seidel, C.; Sundan, A.; Waage, A. Hepatocyte growth factor and its receptor c-met in multiple myeloma. Blood 1996, 88, 3998–4004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewett, D.R.; Vandyke, K.; Lawrence, D.M.; Friend, N.; Noll, J.E.; Geoghegan, J.M.; Croucher, P.I.; Zannettino, A.C.W. DNA barcoding reveals habitual clonal dominance of myeloma plasma cells in the bone marrow microenvironment. Neoplasia 2017, 19, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Lawson, M.A.; McDonald, M.M.; Kovacic, N.; Hua Khoo, W.; Terry, R.L.; Down, J.; Kaplan, W.; Paton-Hough, J.; Fellows, C.; Pettitt, J.A.; et al. Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat. Commun. 2015, 6, 8983. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.J.; Mishima, Y.; Shi, J.; Sklavenitis-Pistofidis, R.; Redd, R.A.; Moschetta, M.; Manier, S.; Roccaro, A.; Sacco, A.; Tai, Y.T.; et al. Progression signature underlies clonal evolution and dissemination of multiple myeloma. Blood 2020. [Google Scholar] [CrossRef] [PubMed]
- Khoo, W.H.; Ledergor, G.; Weiner, A.; Roden, D.L.; Terry, R.L.; McDonald, M.M.; Chai, R.C.; De Veirman, K.; Owen, K.L.; Opperman, K.S.; et al. A niche-dependent myeloid transcriptome signature defines dormant myeloma cells. Blood 2019, 134, 30–43. [Google Scholar] [CrossRef]
- Avet-Loiseau, H.; Li, J.Y.; Facon, T.; Brigaudeau, C.; Morineau, N.; Maloisel, F.; Rapp, M.J.; Talmant, P.; Trimoreau, F.; Jaccard, A.; et al. High incidence of translocations t(11;14)(q13;q32) and t(4;14)(p16;q32) in patients with plasma cell malignancies. Cancer Res. 1998, 58, 5640–5645. [Google Scholar]
- Avet-Loiseau, H.; Daviet, A.; Brigaudeau, C.; Callet-Bauchu, E.; Terre, C.; Lafage-Pochitaloff, M.; Desangles, F.; Ramond, S.; Talmant, P.; Bataille, R. Cytogenetic, interphase, and multicolor fluorescence in situ hybridization analyses in primary plasma cell leukemia: A study of 40 patients at diagnosis, on behalf of the Intergroupe Francophone du Myelome and the Groupe Francais de Cytogenetique Hematologique. Blood 2001, 97, 822–825. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Qi, X.; Yeung, J.; Reece, D.; Xu, W.; Patterson, B. Genetic aberrations including chromosome 1 abnormalities and clinical features of plasma cell leukemia. Leukemia Res. 2009, 33, 259–262. [Google Scholar] [CrossRef]
- Ross, F.M.; Chiecchio, L.; Dagrada, G.; Protheroe, R.K.; Stockley, D.M.; Harrison, C.J.; Cross, N.C.; Szubert, A.J.; Drayson, M.T.; Morgan, G.J.; et al. The t(14;20) is a poor prognostic factor in myeloma but is associated with long-term stable disease in monoclonal gammopathies of undetermined significance. Haematologica 2010, 95, 1221–1225. [Google Scholar] [CrossRef] [Green Version]
- Avet-Loiseau, H.; Malard, F.; Campion, L.; Magrangeas, F.; Sebban, C.; Lioure, B.; Decaux, O.; Lamy, T.; Legros, L.; Fuzibet, J.G.; et al. Translocation t(14;16) and multiple myeloma: Is it really an independent prognostic factor? Blood 2011, 117, 2009–2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, G.; Xu, Y.; Shi, L.; Zou, D.; Deng, S.; Sui, W.; Xie, Z.; Hao, M.; Chang, H.; Qiu, L. t(11;14) multiple myeloma: A subtype associated with distinct immunological features, immunophenotypic characteristics but divergent outcome. Leukemia Res. 2013, 37, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Qin, X.; Acharya, C.; Xu, Y.; Deng, S.; Shi, L.; Zang, M.; Sui, W.; Yi, S.; Li, Z.; et al. Multiple myeloma patients with low proportion of circulating plasma cells had similar survival with primary plasma cell leukemia patients. Ann. Hematol. 2015, 94, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, M.; Agnelli, L.; Fabris, S.; Baldini, L.; Morabito, F.; Bicciato, S.; Verdelli, D.; Intini, D.; Nobili, L.; Cro, L.; et al. Gene expression profiling of plasma cell dyscrasias reveals molecular patterns associated with distinct IGH translocations in multiple myeloma. Oncogene 2005, 24, 2461–2473. [Google Scholar] [CrossRef] [Green Version]
- Zhan, F.; Huang, Y.; Colla, S.; Stewart, J.P.; Hanamura, I.; Gupta, S.; Epstein, J.; Yaccoby, S.; Sawyer, J.; Burington, B.; et al. The molecular classification of multiple myeloma. Blood 2006, 108, 2020–2028. [Google Scholar] [CrossRef] [Green Version]
- Hurt, E.M.; Wiestner, A.; Rosenwald, A.; Shaffer, A.L.; Campo, E.; Grogan, T.; Bergsagel, P.L.; Kuehl, W.M.; Staudt, L.M. Overexpression of c-maf is a frequent oncogenic event in multiple myeloma that promotes proliferation and pathological interactions with bone marrow stroma. Cancer Cell 2004, 5, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Dring, A.M.; Davies, F.E.; Fenton, J.A.; Roddam, P.L.; Scott, K.; Gonzalez, D.; Rollinson, S.; Rawstron, A.C.; Rees-Unwin, K.S.; Li, C.; et al. A global expression-based analysis of the consequences of the t(4;14) translocation in myeloma. Clin. Cancer Res. 2004, 10, 5692–5701. [Google Scholar] [CrossRef] [Green Version]
- Lauring, J.; Abukhdeir, A.M.; Konishi, H.; Garay, J.P.; Gustin, J.P.; Wang, Q.; Arceci, R.J.; Matsui, W.; Park, B.H. The multiple myeloma associated MMSET gene contributes to cellular adhesion, clonogenic growth, and tumorigenicity. Blood 2008, 111, 856–864. [Google Scholar] [CrossRef]
- Lombardi, L.; Poretti, G.; Mattioli, M.; Fabris, S.; Agnelli, L.; Bicciato, S.; Kwee, I.; Rinaldi, A.; Ronchetti, D.; Verdelli, D.; et al. Molecular characterization of human multiple myeloma cell lines by integrative genomics: Insights into the biology of the disease. Genes Chromosomes Cancer 2007, 46, 226–238. [Google Scholar] [CrossRef]
- Neri, P.; Ren, L.; Azab, A.K.; Brentnall, M.; Gratton, K.; Klimowicz, A.C.; Lin, C.; Duggan, P.; Tassone, P.; Mansoor, A.; et al. Integrin β7-mediated regulation of multiple myeloma cell adhesion, migration, and invasion. Blood 2011, 117, 6202. [Google Scholar] [CrossRef] [Green Version]
- Postigo, A.A.; Sanchez-Mateos, P.; Lazarovits, A.I.; Sanchez-Madrid, F.; de Landazuri, M.O. Alpha 4 beta 7 integrin mediates B cell binding to fibronectin and vascular cell adhesion molecule-1. Expression and function of alpha 4 integrins on human B lymphocytes. J. Immunol. 1993, 151, 2471–2483. [Google Scholar] [PubMed]
- Bazan, J.F.; Bacon, K.B.; Hardiman, G.; Wang, W.; Soo, K.; Rossi, D.; Greaves, D.R.; Zlotnik, A.; Schall, T.J. A new class of membrane-bound chemokine with a CX3C motif. Nature 1997, 385, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Umehara, H.; Nakayama, T.; Yoneda, O.; Hieshima, K.; Kakizaki, M.; Dohmae, N.; Yoshie, O.; Imai, T. Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin+/granzyme B+ cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J. Immunol. 2002, 168, 6173–6180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cederblad, L.; Rosengren, B.; Ryberg, E.; Hermansson, N.-O. AZD8797 is an allosteric non-competitive modulator of the human CX3CR1 receptor. Biochem. J. 2016, 473, 641–649. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, R.; Muchtar, E.; Kumar, S.K.; Jevremovic, D.; Buadi, F.K.; Dingli, D.; Dispenzieri, A.; Hayman, S.R.; Hogan, W.J.; Kapoor, P.; et al. Risk stratification in myeloma by detection of circulating plasma cells prior to autologous stem cell transplantation in the novel agent era. Blood Cancer J. 2016, 6, e512. [Google Scholar] [CrossRef] [Green Version]
- Katodritou, E.; Gastari, V.; Verrou, E.; Hadjiaggelidou, C.; Varthaliti, M.; Georgiadou, S.; Laschos, K.; Xirou, P.; Yiannaki, E.; Constantinou, N.; et al. Extramedullary (EMP) relapse in unusual locations in multiple myeloma: Is there an association with precedent thalidomide administration and a correlation of special biological features with treatment and outcome? Leukemia Res. 2009, 33, 1137–1140. [Google Scholar] [CrossRef]
- Lohr, J.G.; Kim, S.; Gould, J.; Knoechel, B.; Drier, Y.; Cotton, M.J.; Gray, D.; Birrer, N.; Wong, B.; Ha, G.; et al. Genetic interrogation of circulating multiple myeloma cells at single-cell resolution. Sci. Transl. Med. 2016, 8, 363ra147. [Google Scholar] [CrossRef] [Green Version]
- Mishima, Y.; Paiva, B.; Shi, J.; Park, J.; Manier, S.; Takagi, S.; Massoud, M.; Perilla-Glen, A.; Aljawai, Y.; Huynh, D.; et al. The mutational landscape of circulating tumor cells in multiple myeloma. Cell Rep. 2017, 19, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Manier, S.; Park, J.; Capelletti, M.; Bustoros, M.; Freeman, S.S.; Ha, G.; Rhoades, J.; Liu, C.J.; Huynh, D.; Reed, S.C.; et al. Whole-exome sequencing of cell-free DNA and circulating tumor cells in multiple myeloma. Nat. Commun. 2018, 9, 1691. [Google Scholar] [CrossRef]
- Rasche, L.; Chavan, S.S.; Stephens, O.W.; Patel, P.H.; Tytarenko, R.; Ashby, C.; Bauer, M.; Stein, C.; Deshpande, S.; Wardell, C.; et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat. Commun. 2017, 8, 268. [Google Scholar] [CrossRef]
- Avet-Loiseau, H.; Attal, M.; Moreau, P.; Charbonnel, C.; Garban, F.; Hulin, C.; Leyvraz, S.; Michallet, M.; Yakoub-Agha, I.; Garderet, L.; et al. Genetic abnormalities and survival in multiple myeloma: The experience of the Intergroupe Francophone du Myelome. Blood 2007, 109, 3489–3495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lode, L.; Eveillard, M.; Trichet, V.; Soussi, T.; Wuilleme, S.; Richebourg, S.; Magrangeas, F.; Ifrah, N.; Campion, L.; Traulle, C.; et al. Mutations in TP53 are exclusively associated with del(17p) in multiple myeloma. Haematologica 2010, 95, 1973–1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, Z.; Zhou, Y.; Zhao, P.; Chen, Y.; Yuan, Y.; Jing, Y.; Wang, X. p53 Deletion promotes myeloma cells invasion by upregulating miR19a/CXCR5. Leukemia Res. 2017, 60, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef] [PubMed]
- Sher, T.; Miller, K.C.; Deeb, G.; Lee, K.; Chanan-Khan, A. Plasma cell leukaemia and other aggressive plasma cell malignancies. Br. J. Haematol. 2010, 150, 418–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peceliunas, V.; Janiulioniene, A.; Matuzeviciene, R.; Zvirblis, T.; Griskevicius, L. Circulating plasma cells predict the outcome of relapsed or refractory multiple myeloma. Leuk Lymphoma 2012, 53, 641–647. [Google Scholar] [CrossRef]
- Keats, J.J.; Chesi, M.; Egan, J.B.; Garbitt, V.M.; Palmer, S.E.; Braggio, E.; Van Wier, S.; Blackburn, P.R.; Baker, A.S.; Dispenzieri, A.; et al. Clonal competition with alternating dominance in multiple myeloma. Blood 2012, 120, 1067–1076. [Google Scholar] [CrossRef]
- Ludwig, H.; Weisel, K.; Petrucci, M.T.; Leleu, X.; Cafro, A.M.; Garderet, L.; Leitgeb, C.; Foa, R.; Greil, R.; Yakoub-Agha, I.; et al. Olaptesed pegol, an anti-CXCL12/SDF-1 Spiegelmer, alone and with bortezomib-dexamethasone in relapsed/refractory multiple myeloma: A Phase IIa Study. Leukemia 2017, 31, 997–1000. [Google Scholar] [CrossRef]
- Hu, J.; Van Valckenborgh, E.; Xu, D.; Menu, E.; De Raeve, H.; De Bruyne, E.; Xu, S.; Van Camp, B.; Handisides, D.; Hart, C.P.; et al. Synergistic induction of apoptosis in multiple myeloma cells by bortezomib and hypoxia-activated prodrug TH-302, in vivo and in vitro. Mol. Cancer Ther. 2013, 12, 1763–1773. [Google Scholar] [CrossRef] [Green Version]
- Laubach, J.P.; Liu, C.J.; Raje, N.S.; Yee, A.J.; Armand, P.; Schlossman, R.L.; Rosenblatt, J.; Hedlund, J.; Martin, M.; Reynolds, C.; et al. A Phase I/II study of evofosfamide, a hypoxia-activated prodrug with or without bortezomib in subjects with relapsed/refractory multiple myeloma. Clin. Cancer Res. 2019, 25, 478–486. [Google Scholar] [CrossRef] [Green Version]



| Receptor on MM PCs | Pro-Disseminatory Factor | Predominant Source in The Myeloma BM | Suggested Role in Dissemination | |||
|---|---|---|---|---|---|---|
| CXCR4 | [33,34,35] | CXCL12 | Hypoxic MM PCs | [35,54] | Mobilisation of MM PCs from the BM | [35,96] |
| Endothelial cells | [29,33,67,68] | Arrest of circulating MM PCs in the BM vasculature | [33,67,68,69] | |||
| BMSCs | [29,30,31,32] | Migration and homing to BM niches | [27,28,76,77] | |||
| CCR1 | [34,35,55] | CCL3 | MM PCs | [35,89,97,98] | Mobilisation of MM PCs from the BM | [35,55] |
| Osteoclasts, macrophages | [86,87,89] | Migration and homing to BM niches | [87,88,89] | |||
| CCR2 | [33,34,87] | CCL2 | Endothelial cells | [33,58] | Migration towards BMECs | [33,58,59] |
| BMSCs, osteoclasts, adipocytes | [32,81,87,90,94] | Migration and homing to BM niches | [33,58] | |||
| CCL7, CCL8 | Osteoclasts | [87] | Migration and homing to BM niches | [90] | ||
| IGF-1R | [99,100] | IGF-1 | Macrophages | [85,87,101] | Migration and homing to BM niches | [84,85] |
| Met | [100,102] | HGF | BMSCs | [79,80,81] | Synergises with CXCL12 to increase MM PC migration | [82] |
| Chromosomal Translocation | Driver Gene | Association with Increased Dissemination 1 | Putative Pro-Disseminatory Factors | |
|---|---|---|---|---|
| t(14;16) and t(14;20) | MAF/MAFB | Increased incidence in PCL and high-PB PC MM | Integrin β7 | [102,103,104,105] |
| CX3CR1 | [114,115] | |||
| IGF-1R | [99,116] | |||
| CCR1 | [99,116] | |||
| t(4;14) | NSD2 | Increased incidence in PCL and high-PB PC MM | EMT-like gene signature (including CDH2, VIM and TWIST1) | [53,66,117,118] |
| IGF-1R | [99,100,116] | |||
| CCR1 | [99,100,116] | |||
| t(11;14) | CCND1 | Increased incidence in PCL | Unclear | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeissig, M.N.; Zannettino, A.C.W.; Vandyke, K. Tumour Dissemination in Multiple Myeloma Disease Progression and Relapse: A Potential Therapeutic Target in High-Risk Myeloma. Cancers 2020, 12, 3643. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12123643
Zeissig MN, Zannettino ACW, Vandyke K. Tumour Dissemination in Multiple Myeloma Disease Progression and Relapse: A Potential Therapeutic Target in High-Risk Myeloma. Cancers. 2020; 12(12):3643. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12123643
Chicago/Turabian StyleZeissig, Mara N., Andrew C. W. Zannettino, and Kate Vandyke. 2020. "Tumour Dissemination in Multiple Myeloma Disease Progression and Relapse: A Potential Therapeutic Target in High-Risk Myeloma" Cancers 12, no. 12: 3643. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers12123643