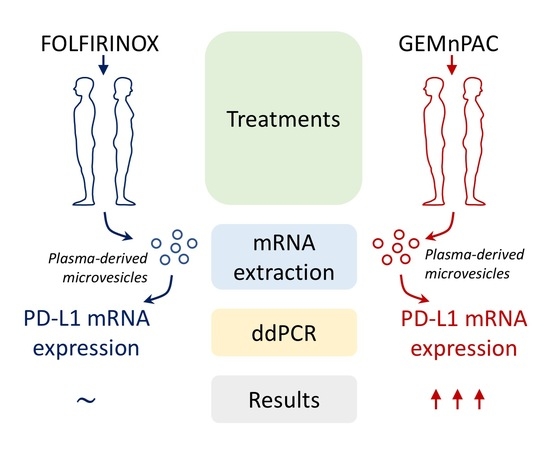
Gemcitabine Plus Nab-Paclitaxel Induces PD-L1 mRNA Expression in Plasma-Derived Microvesicles in Pancreatic Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Selection
2.2. MVs Isolation and Assessment of PD-L1 mRNA Expression
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. PD-L1 mRNA Expression in MVs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; De La Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [Green Version]
- Zeng, S.; Pöttler, M.; Lan, B.; Grützmann, R.; Pilarsky, C.; Yang, H. Chemoresistance in Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 4504. [Google Scholar] [CrossRef] [Green Version]
- Qin, W.; Hu, L.; Zhang, X.; Jiang, S.; Li, J.; Zhang, Z.; Wang, X. The Diverse Function of PD-1/PD-L Pathway Beyond Cancer. Front. Immunol. 2019, 10, 2298. [Google Scholar] [CrossRef]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef] [Green Version]
- Qin, T.; Zeng, Y.-D.; Qin, G.; Xu, F.; Lu, J.-B.; Fang, W.-F.; Xue, C.; Zhan, J.-H.; Zhang, X.-K.; Zheng, Q.-F.; et al. High PD-L1 expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget 2015, 6, 33972–33981. [Google Scholar] [CrossRef] [Green Version]
- Carlsson, J.; Sundqvist, P.; Kosuta, V.; Fält, A.; Giunchi, F.; Fiorentino, M.; Davidsson, S. PD-L1 Expression is Associated with Poor Prognosis in Renal Cell Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Tang, J.; Sun, H.; Zheng, X.; Li, Z.; Sun, T.; Li, J.; Wang, S.; Zhou, X.; Sun, H.; et al. PD-L1 expression as poor prognostic factor in patients with non-squamous non-small cell lung cancer. Oncotarget 2017, 8, 58457–58468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggermont, A.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Mok, T.S.K.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Frontera, O.A.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Yamaki, S.; Yanagimoto, H.; Tsuta, K.; Ryota, H.; Kon, M. PD-L1 expression in pancreatic ductal adenocarcinoma is a poor prognostic factor in patients with high CD8+ tumor-infiltrating lymphocytes: Highly sensitive detection using phosphor-integrated dot staining. Int. J. Clin. Oncol. 2017, 22, 726–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.; Bang, J.-H.; Nam, A.-R.; Park, J.E.; Jin, M.H.; Bang, Y.-J.; Oh, D.-Y. Prognostic implications of soluble programmed death-ligand 1 and its dynamics during chemotherapy in unresectable pancreatic cancer. Sci. Rep. 2019, 9, 11131. [Google Scholar] [CrossRef] [Green Version]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [Green Version]
- Patnaik, A.; Kang, S.P.; Rasco, D.; Papadopoulos, K.P.; Elassaiss-Schaap, J.; Beeram, M.; Drengler, R.; Chen, C.; Smith, L.; Espino, G.; et al. Phase I Study of Pembrolizumab (MK-3475; Anti–PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2015, 21, 4286–4293. [Google Scholar] [CrossRef] [Green Version]
- Nomi, T.; Sho, M.; Akahori, T.; Hamada, K.; Kubo, A.; Kanehiro, H.; Nakamura, S.; Enomoto, K.; Yagita, H.; Azuma, M.; et al. Clinical Significance and Therapeutic Potential of the Programmed Death-1 Ligand/Programmed Death-1 Pathway in Human Pancreatic Cancer. Clin. Cancer Res. 2007, 13, 2151–2157. [Google Scholar] [CrossRef] [Green Version]
- Doi, T.; Ishikawa, T.; Okayama, T.; Oka, K.; Mizushima, K.; Yasuda, T.; Sakamoto, N.; Katada, K.; Uchiyama, K.; Handa, O.; et al. The JAK/STAT pathway is involved in the upregulation of PD-L1 expression in pancreatic cancer cell lines. Oncol. Rep. 2017, 37, 1545–1554. [Google Scholar] [CrossRef] [Green Version]
- Azad, A.; Lim, S.Y.; D’Costa, Z.; Jones, K.; Diana, A.; Sansom, O.J.; Kruger, P.; Liu, S.; McKenna, W.G.; Dushek, O.; et al. PD-L1 blockade enhances response of pancreatic ductal adenocarcinoma to radiotherapy. EMBO Mol. Med. 2017, 9, 167–180. [Google Scholar] [CrossRef]
- Song, X.; Liu, J.; Lu, Y.; Jin, H.; Huang, D. Overexpression of B7-H1 correlates with malignant cell proliferation in pancreatic cancer. Oncol. Rep. 2014, 31, 1191–1198. [Google Scholar] [CrossRef] [Green Version]
- Winograd, R.; Byrne, K.; Evans, R.A.; Odorizzi, P.M.; Meyer, A.R.L.; Bajor, D.L.; Clendenin, C.; Stanger, B.Z.; Furth, E.E.; Wherry, E.J.; et al. Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer Immunol. Res. 2015, 3, 399–411. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Ma, Q.; Chen, X.; Guo, K.; Li, J.; Zhang, M. Clinical Significance of B7-H1 and B7-1 Expressions in Pancreatic Carcinoma. World J. Surg. 2010, 34, 1059–1065. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Zhao, H.; Zhu, D.; Zhi, Q.; Song, S.; Zhang, L.; He, S.; Kuang, Y.; Zhang, Z.; et al. The coexpression and clinical significance of costimulatory molecules B7-H1, B7-H3, and B7-H4 in human pancreatic cancer. Onco Targets Ther. 2014, 7, 1465–1472. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Sun, J.; Wu, H.; Luo, Y.; Wang, L.; Lu, J.; Zhang, Z.; Guo, J.; Liang, Z.; Liu, T. PD-L1 in pancreatic ductal adenocarcinoma: A retrospective analysis of 373 Chinese patients using an in vitro diagnostic assay. Diagn. Pathol. 2018, 13, 5. [Google Scholar] [CrossRef]
- Loos, M.; Giese, N.A.; Kleeff, J.; Giese, T.; Gaida, M.M.; Bergmann, F.; Laschinger, M.; W.Büchler, M.; Friess, H. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett. 2008, 268, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, D.J.; Finetti, P.; Lopresti, A.; Gilabert, M.; Poizat, F.; Turrini, O.; Raoul, J.-L.; Delpero, J.-R.; Moutardier, V.; Birnbaum, D.; et al. Prognostic value of PDL1 expression in pancreatic cancer. Oncotarget 2016, 7, 71198–71210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, T.; Tateishi, A.; Bychkov, A.; Fukuoka, J. Remarkable Alteration of PD-L1 Expression after Immune Checkpoint Therapy in Patients with Non-Small-Cell Lung Cancer: Two Autopsy Case Reports. Int. J. Mol. Sci. 2019, 20, 2578. [Google Scholar] [CrossRef] [Green Version]
- Del Re, M.; Marconcini, R.; Pasquini, G.; Rofi, E.; Vivaldi, C.; Bloise, F.; Restante, G.; Arrigoni, E.; Caparello, C.; Bianco, M.G.; et al. PD-L1 mRNA expression in plasma-derived exosomes is associated with response to anti-PD-1 antibodies in melanoma and NSCLC. Br. J. Cancer 2018, 118, 820–824. [Google Scholar] [CrossRef] [Green Version]
- Del Re, M.; van Schaik, R.H.; Fogli, S.; Mathijssen, R.H.; Cucchiara, F.; Capuano, A.; Scavone, C.; Jenster, G.W.; Danesi, R. Blood-based PD-L1 analysis in tumor-derived extracellular vesicles: Applications for optimal use of anti-PD-1/PD-L1 axis inhibitors. Biochim. Biophys. Acta (BBA) Bioenerg. 2021, 1875, 188463. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef] [Green Version]
- Ng, H.Y.; Li, J.; Tao, L.; Lam, A.K.; Chan, K.W.; Ko, J.M.Y.; Yu, V.Z.; Wong, M.; Li, B.; Lung, M.L. Chemotherapeutic Treatments Increase PD-L1 Expression in Esophageal Squamous Cell Carcinoma through EGFR/ERK Activation. Transl. Oncol. 2018, 11, 1323–1333. [Google Scholar] [CrossRef]
- Gilad, Y.; Eliaz, Y.; Yu, Y.; Han, S.J.; O’Malley, B.W.; Lonard, D.M. Drug-induced PD-L1 expression and cell stress response in breast cancer cells can be balanced by drug combination. Sci. Rep. 2019, 9, 15099. [Google Scholar] [CrossRef] [Green Version]
- Aglietta, M.; Barone, C.; Sawyer, M.B.; Moore, M.J.; Miller, W.H., Jr.; Bagalà, C.; Colombi, F.; Cagnazzo, C.; Gioeni, L.; Wang, E.; et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann. Oncol. 2014, 25, 1750–1755. [Google Scholar] [CrossRef]
- Kamath, S.D.; Kalyan, A.; Kircher, S.; Nimeiri, H.; Fought, A.J.; Benson, A., 3rd; Mulcahy, M. Ipilimumab and Gemcitabine for Advanced Pancreatic Cancer: A Phase Ib Study. Oncologist 2019, 25, e808–e815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, G.J.; Blaydorn, L.; Beck, J.; Bornemann-Kolatzki, K.; Urnovitz, H.; Schütz, E.; Khemka, V. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Investig. New Drugs 2018, 36, 96–102. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Hochster, H.S.; Kim, E.J.-H.; George, B.; Kalyan, A.; Chiorean, E.G.; Waterhouse, D.M.; Gutierrez, M.; Parikh, A.R.; Jain, R.; et al. Phase I study of nivolumab (Nivo) + nab-paclitaxel (nab-P) + gemcitabine (Gem) in advanced pancreatic cancer (APC). J. Clin. Oncol. 2019, 37, 298. [Google Scholar] [CrossRef]
- Moehler, M.; Shitara, K.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Bragagnoli, A.C.; Liu, T.; et al. LBA6_PR Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Ann. Oncol. 2020, 31 (Suppl. 4), S1142–S1215. [Google Scholar] [CrossRef]
- McDaniel, A.S.; Alva, A.; Zhan, T.; Xiao, H.; Cao, X.; Gursky, A.; Siddiqui, J.; Chinnaiyan, A.M.; Jiang, H.; Lee, C.T.; et al. Expression of PDL1 (B7-H1) Before and After Neoadjuvant Chemotherapy in Urothelial Carcinoma. Eur. Urol. Focus 2016, 1, 265–268. [Google Scholar] [CrossRef]
- Gong, W.; Song, Q.; Lu, X.; Gong, W.; Zhao, J.; Min, P.; Yi, X. Paclitaxel Induced B7-H1 Expression in Cancer Cells via the MAPK Pathway. J. Chemother. 2011, 23, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, W.; Yan, Z.; Ma, J.; Zhu, F.; Huo, J. Prognostic value of PD-L1 expression in patients with pancreatic cancer: A PRISMA-compliant meta-analysis. Medicine 2019, 98, e14006. [Google Scholar] [CrossRef]
- Tessier-Cloutier, B.; Kalloger, S.E.; Al-Kandari, M.; Milne, K.; Gao, D.; Nelson, B.H.; Renouf, D.J.; Sheffield, B.S.; Schaeffer, D.F. Programmed cell death ligand 1 cut-point is associated with reduced disease specific survival in resected pancreatic ductal adenocarcinoma. BMC Cancer 2017, 17, 618. [Google Scholar] [CrossRef]
- Kruger, S.; Legenstein, M.-L.; Rösgen, V.; Haas, M.; Modest, D.P.; Westphalen, C.B.; Ormanns, S.; Kirchner, T.; Heinemann, V.; Holdenrieder, S.; et al. Serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death ligand 1 (sPD-L1) in advanced pancreatic cancer. OncoImmunology 2017, 6, e1310358. [Google Scholar] [CrossRef]
- Iwai, T.; Sugimoto, M.; Wakita, D.; Yorozu, K.; Kurasawa, M.; Yamamoto, K. Topoisomerase I inhibitor, irinotecan, depletes regulatory T cells and up-regulates MHC class I and PD-L1 expression, resulting in a supra-additive antitumor effect when combined with anti-PD-L1 antibodies. Oncotarget 2018, 9, 31411–31421. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Wang, Y.; Cheng, X.; Zhu, Y.; Yuan, W.; Dong, J. The Combination Therapy of Fluorouracil and Oxaliplatin Suppress the Progression of Colon Cancer Through miR-183-5p/SOCS3 Axis and Downregulating PD-L1. Cancer Manag. Res. 2021, 13, 1999–2008. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.T.B.; Nasti, A.; Seki, A.; Komura, T.; Inui, H.; Kozaka, T.; Kitamura, Y.; Shiba, K.; Yamashita, T.; Yamashita, T.; et al. Combination of gemcitabine and anti-PD-1 antibody enhances the anticancer effect of M1 macrophages and the Th1 response in a murine model of pancreatic cancer liver metastasis. J. Immunother. Cancer 2020, 8, e001367. [Google Scholar] [CrossRef] [PubMed]
- Del Re, M.; Vivaldi, C.; Rofi, E.; Vasile, E.; Miccoli, M.; Caparello, C.; D’Arienzo, P.D.; Fornaro, L.; Falcone, A.; Danesi, R. Early changes in plasma DNA levels of mutant KRAS as a sensitive marker of response to chemotherapy in pancreatic cancer. Sci. Rep. 2017, 7, 7931. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.D.; Pillarisetty, V.G. T-cell programming in pancreatic adenocarcinoma: A review. Cancer Gene Ther. 2017, 24, 106–113. [Google Scholar] [CrossRef]
- Ricklefs, F.L.; Alayo, Q.; Krenzlin, H.; Mahmoud, A.B.; Speranza, M.C.; Nakashima, H.; Hayes, J.L.; Lee, K.; Balaj, L.; Passaro, C.; et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 2018, 4, eaar2766. [Google Scholar] [CrossRef] [Green Version]
- Lux, A.; Kahlert, C.; Grützmann, R.; Pilarsky, C. c-Met and PD-L1 on Circulating Exosomes as Diagnostic and Prognostic Markers for Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 3305. [Google Scholar] [CrossRef] [Green Version]



| N | % | ||
|---|---|---|---|
| Gender | Male | 25 | 50 |
| Female | 25 | 50 | |
| Age | Median (range) | 60.5 (33–76) years | |
| ECOG PS | 0 | 34 | 68 |
| 1 | 16 | 32 | |
| Chemotherapy | FOLFIRINOX | 16 | 32 |
| GEMnPAC | 34 | 68 | |
| Stage | III | 16 | 32 |
| IV | 34 | 68 | |
| Primary tumor location | Head | 23 | 46 |
| Body-tail | 27 | 54 | |
| Number of metastatic sites | 1–2 | 36 | 72 |
| 3–5 | 14 | 28 | |
| Liver metastases | No | 16 | 32 |
| Yes | 21 | 42 | |
| NA | 13 | 26 | |
| Lung metastases | No | 28 | 56 |
| Yes | 9 | 18 | |
| NA | 13 | 26 | |
| Baseline CA 19-9 (U/mL) | median (range) | 266.6 (0.1–120,000) | |
| ≤median | 25 | 50 | |
| >median | 24 | 48 | |
| NA | 1 | 2 | |
| Baseline NLR | median (range) | 2.21 (0.538–9.46) | |
| ≤median | 24 | 48 | |
| >median | 23 | 46 | |
| NA | 3 | 6 | |
| Baseline PLR | median (range) | 128.99 (60.76–409.9) | |
| ≤median | 24 | 48 | |
| >median | 23 | 46 | |
| NA | 3 | 6 | |
| PD-L1 mRNA at baseline (%FA) | Negative | 22 | 44 |
| Positive | 28 | 56 | |
| PD-L1 mRNA at 3 months (%FA) | Negative | 11 | 22 |
| Positive | 38 | 76 | |
| NA | 1 | 2 |
| GEMnPAC Group | FOLFIRINOX Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | PD-L1 FA (Baseline) | PD-L1 FA (3 Months) | PD-L1 FA Change | Tumor Response | Patient | PD-L1 FA (Baseline) | PD-L1 FA (3 Months) | PD-L1 FA Change | Tumor Response |
| 1 | 0.007 | 0.005 | ↓ | PR | 35 | 0.007 | 0.004 | ↓ | SD |
| 2 | 0 | 0.005 | ↑ | SD | 36 | 0.018 | 0.007 | ↓ | PR |
| 3 | 0 | 0 | = | PR | 37 | 0 | 0 | = | PD |
| 4 | 0.007 | 0.014 | ↑ | SD | 38 | 0 | 0.007 | ↑ | PD |
| 5 | 0.004 | 0.007 | ↑ | SD | 39 | 0.001 | 0 | ↓ | SD |
| 6 | 0.009 | 0.009 | = | PD | 40 | 0 | 0.009 | ↑ | SD |
| 7 | 0.002 | 0.012 | ↑ | PR | 41 | 0 | 0 | = | SD |
| 8 | 0 | 0.002 | ↑ | PD | 42 | 0 | 0 | = | SD |
| 9 | 0.005 | 0.003 | ↓ | PD | 43 | 0.009 | 0.003 | ↓ | PR |
| 10 | 0 | 0.017 | ↑ | PD | 44 | 0 | 0.009 | ↑ | PD |
| 11 | 0.002 | 0.008 | ↑ | SD | 45 | 0.007 | 0.006 | ↓ | SD |
| 12 | 0.003 | 0.005 | ↑ | SD | 46 | 0.017 | 0.006 | ↓ | SD |
| 13 | 0.005 | 0.003 | ↓ | SD | 47 | 0.006 | 0.01 | ↑ | SD |
| 14 | 0.015 | 0.003 | ↓ | SD | 48 | 0.005 | 0.006 | ↑ | SD |
| 15 | 0.004 | 0 | ↓ | SD | 49 | 0.003 | 0.003 | = | PD |
| 16 | 0.008 | 0.004 | ↓ | PD | 50 | 0.004 | 0.004 | = | SD |
| 17 | 0 | 0 | = | PR | |||||
| 18 | 0.008 | 0.006 | ↓ | SD | |||||
| 19 | 0 | 0.018 | ↑ | SD | |||||
| 20 | 0 | 0 | = | PD | |||||
| 21 | 0 | 0.016 | ↑ | PR | |||||
| 22 | 0.001 | 0 | ↓ | PD | |||||
| 23 | 0 | 0.004 | ↑ | PR | |||||
| 24 | 0 | 0.009 | ↑ | SD | |||||
| 25 | 0 | 0.019 | ↑ | SD | |||||
| 26 | 0 | 0 | = | SD | |||||
| 27 | 0 | 0.011 | ↑ | PR | |||||
| 28 | 0 | 0.003 | ↑ | PR | |||||
| 29 | 0.003 | 0.001 | ↓ | PR | |||||
| 30 | 0.002 | 0.015 | ↑ | PD | |||||
| 31 | 0 | 0 | = | SD | |||||
| 32 | 0 | 0.005 | ↑ | SD | |||||
| 33 | 0.004 | 0.006 | ↑ | SD | |||||
| 34 | 0.005 | NE | NE | PD | |||||
| PD-L1 mRNA FA (Median, %) | p-Value | ||
|---|---|---|---|
| Age | <60.5 vs. ≥60.5 | 0.00125 vs. 0.00243 | 0.83 |
| Gender | Male vs. Female | 0 vs. 0.00272 | 0.43 |
| Stage | III vs. IV | 0.00505 vs. 0.00347 | 0.06 |
| ECOG PS | 0 vs. 1 | 0.00257 vs. 0.0011 | 0.44 |
| Primary tumor location | Body-tail vs. Head | 0 vs. 0.00367 | 0.14 |
| Baseline CA 19-9 | ≤median 266.6 vs. >median 266.6 | 0.00359 vs. 0 | 0.29 |
| Baseline NLR | ≤median 2.21 vs. >median 2.21 | 0.0031 vs. 0.000693 | 0.39 |
| Baseline PLR | ≤median 128.99 vs. >median 128.99 | 0.00173 vs. 0.00243 | 0.44 |
| Number of metastatic sites | 1–2 vs. 3–5 | 0.000973 vs. 0.003 | 0.43 |
| Liver metastases | Yes vs. No | 0.000693 vs. 0.00287 | 0.38 |
| Lung metastases | Yes vs. No | 0 vs. 0.00173 | 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Re, M.; Vivaldi, C.; Rofi, E.; Salani, F.; Crucitta, S.; Catanese, S.; Fontanelli, L.; Massa, V.; Cucchiara, F.; Fornaro, L.; et al. Gemcitabine Plus Nab-Paclitaxel Induces PD-L1 mRNA Expression in Plasma-Derived Microvesicles in Pancreatic Cancer. Cancers 2021, 13, 3738. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13153738
Del Re M, Vivaldi C, Rofi E, Salani F, Crucitta S, Catanese S, Fontanelli L, Massa V, Cucchiara F, Fornaro L, et al. Gemcitabine Plus Nab-Paclitaxel Induces PD-L1 mRNA Expression in Plasma-Derived Microvesicles in Pancreatic Cancer. Cancers. 2021; 13(15):3738. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13153738
Chicago/Turabian StyleDel Re, Marzia, Caterina Vivaldi, Eleonora Rofi, Francesca Salani, Stefania Crucitta, Silvia Catanese, Lorenzo Fontanelli, Valentina Massa, Federico Cucchiara, Lorenzo Fornaro, and et al. 2021. "Gemcitabine Plus Nab-Paclitaxel Induces PD-L1 mRNA Expression in Plasma-Derived Microvesicles in Pancreatic Cancer" Cancers 13, no. 15: 3738. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13153738