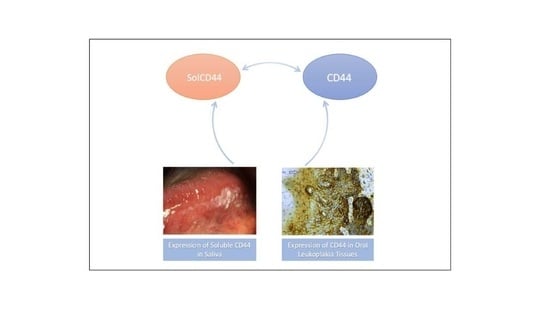
Correlation of Soluble CD44 Expression in Saliva and CD44 Protein in Oral Leukoplakia Tissues
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Oral Rinse
2.3. Microscopic and Immunohistochemical Examination
2.4. Statistical Analysis
3. Results
3.1. Clinical, Morphological, and Salivary SolCD44 and Total Protein Characteristics
3.2. Immunohistochemical CD44 Antigen Characteristics
3.3. Immunohistochemical CD9 Antigen Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Diz, P.; Meleti, M.; Diniz-Freitas, M.; Vescovi, P.; Warnakulasuriya, S.; Johnson, N.W.; Kerr, A.R. Oral and pharyngeal cancer in Europe: Incidence, mortality and trends as presented to the Global Oral Cancer Forum. Transl. Res. Oral Oncol. 2017, 2, 2057178X17701517. [Google Scholar] [CrossRef] [Green Version]
- Roza, A.L.O.C.; Kowalski, L.P.; William, W.N.; de Castro, G.; Chaves, A.L.F.; Araújo, A.L.D.; Ribeiro, A.C.P.; Brandão, T.B.; Lopes, M.A.; Vargas, P.A.; et al. Oral leukoplakia and erythroplakia in young patients: A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Petti, S. Pooled estimate of world leukoplakia prevalence: A systematic review. Oral Oncol. 2003, 39, 770–780. [Google Scholar] [CrossRef]
- Mello, F.W.; Miguel, A.F.P.; Dutra, K.L.; Porporatti, A.L.; Warnakulasuriya, S.; Guerra, E.N.S.; Rivero, E.R.C. Prevalence of oral potentially malignant disorders: A systematic review and meta-analysis. J. Oral Pathol. Med. 2018, 47, 633–640. [Google Scholar] [CrossRef]
- Brouns, E.R.; Baart, J.A.; Bloemena, E.; Karagozoglu, H.; van der Waal, I. The relevance of uniform reporting in oral leukoplakia: Definition, certainty factor and staging based on experience with 275 patients. Med. Oral Patol. Oral Cir. Bucal. 2013, 18, e19–e26. [Google Scholar] [CrossRef]
- Aguirre-Urizar, J.M.; Lafuente-Ibáñez de Mendoza, I.; Warnakulasuriya, S. Malignant transformation of oral leukoplakia: Systematic review and meta-analysis of the last 5 years. Oral Dis. 2021, 27, 1881–1895. [Google Scholar] [CrossRef]
- Arduino, P.G.; Bagan, J.; El-Naggar, A.K.; Carrozzo, M. Urban legends series: Oral leukoplakia. Oral Dis. 2013, 19, 642–659. [Google Scholar] [CrossRef]
- Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; Pardiñas López, S.; Shanti, R.M. Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic review and meta-analysis of malignant transformation rate by subtype. Head Neck 2020, 42, 539–555. [Google Scholar] [CrossRef]
- Van der Waal, I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 2009, 45, 317–323. [Google Scholar] [CrossRef]
- Kannan, S.; Chandran, G.J.; Pillai, K.R.; Mathew, B.; Sujathan, K.; Nalinakumary, K.R.; Nair, M.K. Expression of p53 in leukoplakia and squamous cell carcinoma of the oral mucosa: Correlation with expression of Ki67. Clin. Mol. Pathol. 1996, 49, M170–M175. [Google Scholar] [CrossRef] [Green Version]
- Pitiyage, G.; Tilakaratne, W.M.; Tavassoli, M.; Warnakulasuriya, S. Molecular markers in oral epithelial dysplasia: Review. J. Oral Pathol. Med. 2009, 38, 737–752. [Google Scholar] [CrossRef]
- Nasser, W.; Flechtenmacher, C.; Holzinger, D.; Hofele, C.; Bosch, F.X. Aberrant expression of p53, p16INK4a and Ki-67 as basic biomarker for malignant progression of oral leukoplakias. J. Oral Pathol. Med. 2011, 40, 629–635. [Google Scholar] [CrossRef]
- Monteiro, L.; Mello, F.W.; Warnakulasuriya, S. Tissue biomarkers for predicting the risk of oral cancer in patients diagnosed with oral leukoplakia: A systematic review. Oral Dis. 2020, 27, 1977–1992. [Google Scholar] [CrossRef]
- Kitahara, A.B.P.; Michels, A.C.; Luiz, S.T.; Nagashima, S.; Camargo Martins, A.P.; de Azevedo, M.L.V.; Azevedo Alanis, L.R.; Couto Souza, P.H.; Ignácio, S.A.; de Noronha, L.; et al. Immunohistochemical detection of NANOG in oral leukoplakia. Oral Dis. 2021. [Google Scholar] [CrossRef]
- Liu, W.; Yao, Y.; Shi, L.; Tang, G.; Wu, L. A novel lncRNA LOLA1 may predict malignant progression and promote migration, invasion, and EMT of oral leukoplakia via the AKT/GSK-3β pathway. J. Cell Biochem. 2021, 122, 1302–1312. [Google Scholar] [CrossRef]
- Dzwonek, J.; Wilczynski, G.M. CD44: Molecular interactions, signaling and functions in the nervous system. Front. Cell Neurosci. 2015, 9, 175. [Google Scholar] [CrossRef] [Green Version]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef] [Green Version]
- Thorne, R.F.; Legg, J.W.; Isacke, C.M. The role of the CD44 transmembrane and cytoplasmic domains in co-ordinating adhesive and signalling events. J. Cell Sci. 2004, 117, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Prince, M.E.; Sivanandan, R.; Kaczorowski, A.; Wolf, G.T.; Kaplan, M.J.; Dalerba, P.; Weissman, I.L.; Clarke, M.F.; Ailles, L.E. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 973–978. [Google Scholar] [CrossRef] [Green Version]
- Emich, H.; Chapireau, D.; Hutchison, I.; Mackenzie, I. The potential of CD44 as a diagnostic and prognostic tool in oral cancer. J. Oral Pathol. Med. 2015, 44, 393–400. [Google Scholar] [CrossRef]
- Thapa, R.; Wilson, G.D. The Importance of CD44 as a Stem Cell Biomarker and Therapeutic Target in Cancer. Stem Cells Int. 2016, 2016, 2087204. [Google Scholar] [CrossRef] [Green Version]
- Venkat Naga, S.K.S.; Shekar, P.C.; Kattappagari, K.K.; Prakash Chandra, K.L.; Reddy, G.S.; Ramana Reddy, B.V. Expression of cluster differentiation-44 stem cell marker in grades of oral epithelial dysplasia: A preliminary study. J. Oral Maxillofac. Pathol. 2019, 23, 203–207. [Google Scholar] [CrossRef]
- Chen, D.; Wang, C.Y. Targeting cancer stem cells in squamous cell carcinoma. Precis. Clin. Med. 2019, 2, 152–165. [Google Scholar] [CrossRef]
- Skandalis, S.S.; Karalis, T.T.; Chatzopoulos, A.; Karamanos, N.K. Hyaluronan-CD44 axis orchestrates cancer stem cell functions. Cell Signal. 2019, 63, 109377. [Google Scholar] [CrossRef]
- Wang, S.J.; Wong, G.; de Heer, A.M.; Xia, W.; Bourguignon, L.Y. CD44 variant isoforms in head and neck squamous cell carcinoma progression. Laryngoscope 2009, 119, 1518–1530. [Google Scholar] [CrossRef]
- Mack, B.; Gires, O. CD44s and CD44v6 expression in head and neck epithelia. PLoS ONE 2008, 3, e3360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godge, P.Y.; Poonja, L.S. Quantitative assessment of expression of cell adhesion molecule (CD44) splice variants: CD44 standard (CD44s) and v5, v6 isoforms in oral leukoplakias: An immunohistochemical study. Indian J. Dent. Res. 2011, 22, 493–494. [Google Scholar] [CrossRef] [PubMed]
- Wood, H.M.; Daly, C.; Chalkley, R.; Senguven, B.; Ross, L.; Egan, P.; Chengot, P.; Graham, J.; Sethi, N.; Ong, T.K.; et al. The genomic road to invasion-examining the similarities and differences in the genomes of associated oral pre-cancer and cancer samples. Genome Med. 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, L.A.; Mussavira, S.; Bindhu, O.S. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: A systematic review. Biochem. Med. 2015, 25, 177–192. [Google Scholar] [CrossRef]
- Rapado-González, Ó.; Martínez-Reglero, C.; Salgado-Barreira, Á.; Takkouche, B.; López-López, R.; Suárez-Cunqueiro, M.M.; Muinelo-Romay, L. Salivary biomarkers for cancer diagnosis: A meta-analysis. Ann. Med. 2020, 52, 131–144. [Google Scholar] [CrossRef]
- Ghallab, N.; Shaker, O. Salivary-soluble CD44 levels in smokers and non-smokers with chronic periodontitis: A pilot study. J. Periodontol. 2010, 81, 710–717. [Google Scholar] [CrossRef]
- Chaiyarit, P.; Thongprasom, K.; Satayut, S.; Dhanuthai, K.; Piboonratanakit, P.; Phothipakdee, P.; Subarnbhesaj, A.; Limlertmongkol, S.; Chaimusig, M. Alteration of the expression of CD44 [corrected] isoforms in oral epithelia and saliva from patients with oral lichen planus. J. Clin. Immunol. 2008, 28, 26–34. [Google Scholar] [CrossRef]
- Kaur, S.; Narayanswamy, S.; Ramesh, A.V. Comparative evaluation of salivary soluble CD44 levels in periodontal health and disease. J. Indian Soc. Periodontol. 2014, 18, 734–738. [Google Scholar] [CrossRef]
- Gualtero, D.F.; Suarez Castillo, A. Biomarkers in saliva for the detection of oral squamous cell carcinoma and their potential use for early diagnosis: A systematic review. Acta Odontol. Scand. 2016, 74, 170–177. [Google Scholar] [CrossRef]
- Pereira, L.H.; Reis, I.M.; Reategui, E.P.; Gordon, C.; Saint-Victor, S.; Duncan, R.; Gomez, C.; Bayers, S.; Fisher, P.; Perez, A.; et al. Risk Stratification System for Oral Cancer Screening. Cancer Prev. Res. 2016, 9, 445–455. [Google Scholar] [CrossRef] [Green Version]
- Franzmann, E.J.; Reategui, E.P.; Pereira, L.H.; Pedroso, F.; Joseph, D.; Allen, G.O.; Hamilton, K.; Reis, I.; Duncan, R.; Goodwin, W.J.; et al. Salivary protein and solCD44 levels as a potential screening tool for early detection of head and neck squamous cell carcinoma. Head Neck 2012, 34, 687–695. [Google Scholar] [CrossRef] [Green Version]
- Allegra, E.; Trapasso, S.; La Boria, A.; Aragona, T.; Pisani, D.; Belfiore, A.; Garozzo, A. Prognostic role of salivary CD44sol levels in the follow-up of laryngeal carcinomas. J. Oral Pathol. Med. 2014, 43, 276–281. [Google Scholar] [CrossRef]
- Trapasso, S.; Garozzo, A.; Belfiore, A.; Allegra, E. Evaluation of the CD44 isoform v-6 (sCD44var, v6) in the saliva of patients with laryngeal carcinoma and its prognostic role. Cancer Biomark. 2016, 16, 275–280. [Google Scholar] [CrossRef]
- Dasari, S.; Rajendra, W.; Valluru, L. Evaluation of soluble CD44 protein marker to distinguish the premalignant and malignant carcinoma cases in cervical cancer patients. Med. Oncol. 2014, 31, 139. [Google Scholar] [CrossRef]
- Sawant, S.; Ahire, C.; Dongre, H.; Joshi, S.; Jamghare, S.; Rane, P.; Kane, S.; Chaukar, D. Prognostic significance of elevated serum CD44 levels in patients with oral squamous cell carcinoma. J. Oral Pathol. Med. 2018, 47, 665–673. [Google Scholar] [CrossRef]
- García-Posadas, L.; Contreras-Ruiz, L.; López-García, A.; Villarón Álvarez, S.; Maldonado, M.J.; Diebold, Y. Hyaluronan receptors in the human ocular surface: A descriptive and comparative study of RHAMM and CD44 in tissues, cell lines and freshly collected samples. Histochem. Cell Biol. 2012, 137, 165–176. [Google Scholar] [CrossRef]
- Franzmann, E.J.; Reategui, E.P.; Pedroso, F.; Pernas, F.G.; Karakullukcu, B.M.; Carraway, K.L.; Hamilton, K.; Singal, R.; Goodwin, W.J. Soluble CD44 is a potential marker for the early detection of head and neck cancer. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1348–1355. [Google Scholar] [CrossRef] [Green Version]
- Franzmann, E.J.; Donovan, M.J. Effective early detection of oral cancer using a simple and inexpensive point of care device in oral rinses. Expert Rev. Mol. Diagn. 2018, 18, 837–844. [Google Scholar] [CrossRef]
- Sivadasan, P.; Gupta, M.K.; Sathe, G.J.; Balakrishnan, L.; Palit, P.; Gowda, H.; Suresh, A.; Kuriakose, M.A.; Sirdeshmukh, R. Human salivary proteome—A resource of potential biomarkers for oral cancer. J. Proteom. 2015, 127, 89–95. [Google Scholar] [CrossRef]
- Jai Kumar, S.K.; Gayathri, R.; Priya, V.V. Evaluation of salivary total proteins, albumin, globulin, and albumin/globulin ratio among healthy individuals and patients with chronic gingivitis. Drug Invent. Today 2018, 10, 925–928. [Google Scholar]
- Castagnola, M.; Cabras, T.; Iavarone, F.; Vincenzoni, F.; Vitali, A.; Pisano, E.; Nemolato, S.; Scarano, E.; Fiorita, A.; Vento, G.; et al. Top-down platform for deciphering the human salivary proteome. J. Matern. Fetal Neonatal Med. 2012, 25, 27–43. [Google Scholar] [CrossRef] [Green Version]
- Peng, Q.; Yang, J.Y.; Zhou, G. Emerging functions and clinical applications of exosomes in human oral diseases. Cell Biosci. 2020, 10, 68. [Google Scholar] [CrossRef]
- Zlotogorski-Hurvitz, A.; Dayan, D.; Chaushu, G.; Korvala, J.; Salo, T.; Sormunen, R.; Vered, M. Human saliva-derived exosomes: Comparing methods of isolation. J. Histochem. Cytochem. 2015, 63, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Chen, J.Q.; Liu, J.L.; Tian, L. Exosomes in tumor microenvironment: Novel transporters and biomarkers. J. Transl. Med. 2016, 14, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huan, J.; Gao, Y.; Xu, J.; Sheng, W.; Zhu, W.; Zhang, S.; Cao, J.; Ji, J.; Zhang, L.; Tian, Y. Overexpression of CD9 correlates with tumor stage and lymph node metastasis in esophageal squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 3054–3061. [Google Scholar] [PubMed]
- Menck, K.; Sivaloganathan, S.; Bleckmann, A.; Binder, C. Microvesicles in Cancer: Small Size, Large Potential. Int. J. Mol. Sci. 2020, 21, 5373. [Google Scholar] [CrossRef]
- Fontana, F.; Carollo, E.; Melling, G.E.; Carter, D.R.F. Extracellular Vesicles: Emerging Modulators of Cancer Drug Resistance. Cancers 2021, 13, 749. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Z.; Shang, Z.; Sun, K.; Niu, X.; Qian, L.; Fan, L.Y.; Cao, C.X.; Xiao, H. Facile preparation of salivary extracellular vesicles for cancer proteomics. Sci. Rep. 2016, 6, 24669. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Jia, L.; Zheng, Y.; Li, W. Salivary Exosomes: Emerging Roles in Systemic Disease. Int. J. Biol. Sci. 2018, 14, 633–643. [Google Scholar] [CrossRef]
- Kusukawa, J.; Ryu, F.; Kameyama, T.; Mekada, E. Reduced expression of CD9 in oral squamous cell carcinoma: CD9 expression inversely related to high prevalence of lymph node metastasis. J. Oral Pathol. Med. 2001, 30, 73–79. [Google Scholar] [CrossRef]
- Buim, M.E.; Lourenço, S.V.; Carvalho, K.C.; Cardim, R.; Pereira, C.; Carvalho, A.L.; Fregnani, J.H.; Soares, F.A. Downregulation of CD9 protein expression is associated with aggressive behavior of oral squamous cell carcinoma. Oral Oncol. 2010, 46, 166–171. [Google Scholar] [CrossRef]
- Hirano, C.; Nagata, M.; Noman, A.A.; Kitamura, N.; Ohnishi, M.; Ohyama, T.; Kobayashi, T.; Suzuki, K.; Yoshizawa, M.; Izumi, N.; et al. Tetraspanin gene expression levels as potential biomarkers for malignancy of gingival squamous cell carcinoma. Int. J. Cancer 2009, 124, 2911–2916. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, J.; Chen, W. M1-like tumor-associated macrophages activated by exosome-transferred THBS1 promote malignant migration in oral squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2018, 37, 143. [Google Scholar] [CrossRef] [Green Version]
- Nankivell, P.; Williams, H.; McConkey, C.; Webster, K.; High, A.; MacLennan, K.; Senguven, B.; Rabbitts, P.; Mehanna, H. Tetraspanins CD9 and CD151, epidermal growth factor receptor and cyclooxygenase-2 expression predict malignant progression in oral epithelial dysplasia. Br. J. Cancer 2013, 109, 2864–2874. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Han, Y.; Zhao, Z.; Ji, X.; Wang, X.; Jin, J.; Wang, Q.; Guo, X.; Cheng, Z.; Lu, M.; et al. Oral mucosal mesenchymal stem cell-derived exosomes: A potential therapeutic target in oral premalignant lesions. Int. J. Oncol. 2019, 54, 1567–1578. [Google Scholar] [CrossRef] [Green Version]
- Yap, T.; Pruthi, N.; Seers, C.; Belobrov, S.; McCullough, M.; Celentano, A. Extracellular Vesicles in Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 1197. [Google Scholar] [CrossRef] [Green Version]
- Tobón-Arroyave, S.I.; Celis-Mejía, N.; Córdoba-Hidalgo, M.P.; Isaza-Guzmán, D.M. Decreased salivary concentration of CD9 and CD81 exosome-related tetraspanins may be associated with the periodontal clinical status. J. Clin. Periodontol. 2019, 46, 470–480. [Google Scholar] [CrossRef]
- Chaparro Padilla, A.; Weber Aracena, L.; Realini Fuentes, O.; Albers Busquetts, D.; Hernández Ríos, M.; Ramírez Lobos, V.; Pascual La Rocca, A.; Nart Molina, J.; Beltrán Varas, V.; Acuña-Gallardo, S.; et al. Molecular signatures of extracellular vesicles in oral fluids of periodontitis patients. Oral Dis. 2020, 26, 1318–1325. [Google Scholar] [CrossRef]
- Zhong, W.Q.; Ren, J.G.; Xiong, X.P.; Man, Q.W.; Zhang, W.; Gao, L.; Li, C.; Liu, B.; Sun, Z.J.; Jia, J.; et al. Increased salivary microvesicles are associated with the prognosis of patients with oral squamous cell carcinoma. J. Cell. Mol. Med. 2019, 23, 4054–4062. [Google Scholar] [CrossRef]
- Dzudzilo, M.; Kleina, R.; Čēma, I.; Dabuzinskiene, A.; Svirskis, Š. Expression and Localisation of CD44 Antigen as a Prognostic Factor of Oral Leukoplakia. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2021, 75, 68–74. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Johnson, N.W.; van der Waal, I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J. Oral Pathol. Med. 2007, 36, 575–580. [Google Scholar] [CrossRef]
- Neville, B.W.; Day, T.A. Oral cancer and precancerous lesions. CA Cancer J. Clin. 2002, 52, 195–215. [Google Scholar] [CrossRef]
- Cerqueira, J.M.; Pontes, F.S.; Santos-Silva, A.R.; Almeida, O.P.; Costa, R.F.; Fonseca, F.P.; Gomez, R.S.; Neto, N.C.; Miyahara, L.A.; Rodrigues-Fernandes, C.I.; et al. Malignant transformation of oral leukoplakia: A multicentric retrospective study in Brazilian population. Med. Oral Patol. Oral Cir. Bucal. 2021, 26, e292–e298. [Google Scholar] [CrossRef]
- De Azevedo, A.B.; Dos Santos, T.C.R.B.; Lopes, M.A.; Pires, F.R. Oral leukoplakia, leukoerythroplakia, erythroplakia and actinic cheilitis: Analysis of 953 patients focusing on oral epithelial dysplasia. J. Oral Pathol. Med. 2021, 50, 829–840. [Google Scholar] [CrossRef]
- Van der Waal, I. Historical perspective and nomenclature of potentially malignant or potentially premalignant oral epithelial lesions with emphasis on leukoplakia-some suggestions for modifications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 577–581. [Google Scholar] [CrossRef] [Green Version]
- Nagao, T.; Warnakulasuriya, S.; Hasegawa, S.; Sakuma, H.; Miyabe, S.; Komaki, K.; Ishii, K.; Machida, J.; Kimura, M.; Kuroyanagi, N.; et al. Elucidating risk factors for oral leukoplakia affecting gingivae in Japanese subjects. Transl. Res. Oral Oncol. 2016, 1, 2057178X16654704. [Google Scholar] [CrossRef] [Green Version]
- Kramer, I.R.; Lucas, R.B.; Pindborg, J.J.; Sobin, L.H. Definition of leukoplakia and related lesions: An aid to studies on oral precancer. Oral Surg. Oral Med. Oral Pathol. 1978, 46, 518–539. [Google Scholar]
- Warnakulasuriya, S. Clinical features and presentation of oral potentially malignant disorders. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 582–590. [Google Scholar] [CrossRef] [Green Version]
- Yagyuu, T.; Funayama, N.; Imada, M.; Kirita, T. Effect of smoking status and programmed death-ligand 1 expression on the microenvironment and malignant transformation of oral leukoplakia: A retrospective cohort study. PLoS ONE 2021, 16, e0250359. [Google Scholar] [CrossRef]
- Bánóczy, J.; Squier, C.A.; Kremer, M.; Wertz, P.W.; Kövesi, G.; Szende, B.; Dombi, C. The permeability of oral leukoplakia. Eur. J. Oral Sci. 2003, 111, 312–315. [Google Scholar] [CrossRef]
- Collins, L.M.; Dawes, C. The surface area of the adult human mouth and thickness of the salivary film covering the teeth and oral mucosa. J. Dent. Res. 1987, 66, 1300–1302. [Google Scholar] [CrossRef]
- Abati, S.; Bramati, C.; Bondi, S.; Lissoni, A.; Trimarchi, M. Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis. Int. J. Environ. Res. Public Health 2020, 17, 9160. [Google Scholar] [CrossRef]
- Lesch, C.A.; Squier, C.A.; Cruchley, A.; Williams, D.M.; Speight, P. The permeability of human oral mucosa and skin to water. J. Dent. Res. 1989, 68, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Martorell-Calatayud, A.; Botella-Estrada, R.; Bagán-Sebastián, J.V.; Sanmartín-Jiménez, O.; Guillén-Barona, C. Oral leukoplakia: Clinical, histopathologic, and molecular features and therapeutic approach. Actas Dermosifiliogr. 2009, 100, 669–684. [Google Scholar] [CrossRef]
- Oliveira, D.T.; Odell, E.W. Expression of CD44 variant exons by normal oral epithelia. Oral Oncol. 1997, 33, 260–262. [Google Scholar] [CrossRef]
- Ioachim, E.; Assimakopoulos, D.; Goussia, A.C.; Peschos, D.; Skevas, A.; Agnantis, N.J. Glycoprotein CD44 expression in benign, premalignant and malignant epithelial lesions of the larynx: An immunohistochemical study including correlation with Rb, p53, Ki-67 and PCNA. Histol. Histopathol. 1999, 14, 1113–1118. [Google Scholar] [CrossRef]
- Rautava, J.; Soukka, T.; Inki, P.; Leimola-Virtanen, R.; Saloniemi, I.; Happonen, R.P.; Heikinheimo, K. CD44v6 in developing, dysplastic and malignant oral epithelia. Oral Oncol. 2003, 39, 373–379. [Google Scholar] [CrossRef]
- Groma, V.; Kazanceva, A.; Nora-Krukle, Z.; Murovska, M. Oropharyngeal malignant epithelial cell, lymphocyte and macrophage CD44 surface receptors for hyaluronate are expressed in sustained EBV infection: Immunohistochemical data and EBV DNA tissue indices. Pathol. Res. Pract. 2012, 208, 518–526. [Google Scholar] [CrossRef]
- Harada, H.; Takahashi, M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J. Biol. Chem. 2007, 282, 5597–5607. [Google Scholar] [CrossRef] [Green Version]
- Ensinck, M.A.; Valles, M.; Lebensohn, N.; Cotorruelo, C.; Biondi, C. Expression of the FUT2 gene and CD44 marker in patients with oral lesions. Inmunología 2013, 32, 123–128. [Google Scholar] [CrossRef]
- Miletti-González, K.E.; Murphy, K.; Kumaran, M.N.; Ravindranath, A.K.; Wernyj, R.P.; Kaur, S.; Miles, G.D.; Lim, E.; Chan, R.; Chekmareva, M.; et al. Identification of function for CD44 intracytoplasmic domain (CD44-ICD): Modulation of matrix metalloproteinase 9 (MMP-9) transcription via novel promoter response element. J. Biol. Chem. 2012, 287, 18995–19007. [Google Scholar] [CrossRef] [Green Version]
- Ghazi, N.; Saghravanian, N.; Ghazi, A.; Shakeri, M.; Khajehbahrami, H. CD44 Expression in Dysplastic and Non-Dysplastic Oral Lichen Planus. Int. J. Cancer Manag. 2020, in press. [Google Scholar] [CrossRef]
- Cohen, E.R.; Reis, I.M.; Gomez-Fernandez, C.; Smith, D.; Pereira, L.; Freiser, M.E.; Marotta, G.; Thomas, G.R.; Sargi, Z.B.; Franzmann, E.J. CD44 and associated markers in oral rinses and tissues from oral and oropharyngeal cancer patients. Oral Oncol. 2020, 106, 104720. [Google Scholar] [CrossRef]
- Wang, L.; Yin, P.; Wang, J.; Wang, Y.; Sun, Z.; Zhou, Y.; Guan, X. Delivery of mesenchymal stem cells-derived extracellular vesicles with enriched miR-185 inhibits progression of OPMD. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2481–2491. [Google Scholar] [CrossRef] [Green Version]
- Ralhan, R.; Desouza, L.V.; Matta, A.; Tripathi, S.C.; Ghanny, S.; Dattagupta, S.; Thakar, A.; Chauhan, S.S.; Siu, K.W. iTRAQ-multidimensional liquid chromatography and tandem mass spectrometry-based identification of potential biomarkers of oral epithelial dysplasia and novel networks between inflammation and premalignancy. J. Proteome Res. 2009, 8, 300–309. [Google Scholar] [CrossRef]
- Sivadasan, P.; Gupta, M.K.; Sathe, G.; Sudheendra, H.V.; Sunny, S.P.; Renu, D.; Hari, P.S.; Gowda, H.; Suresh, A.; Kuriakose, M.A.; et al. Salivary proteins from dysplastic leukoplakia and oral squamous cell carcinoma and their potential for early detection. J. Proteom. 2020, 212, 103574. [Google Scholar] [CrossRef]
- Metgud, R.; Patel, S. Serum and salivary levels of albumin as diagnostic tools for oral pre-malignancy and oral malignancy. Biotech. Histochem. 2014, 89, 8–13. [Google Scholar] [CrossRef]
- Aponte, P.M.; Caicedo, A. Stemness in Cancer: Stem Cells, Cancer Stem Cells, and Their Microenvironment. Stem Cells Int. 2017, 2017, 5619472. [Google Scholar] [CrossRef]










| Patient | Age | Gender | Localization | Clinical Type of Leukoplakia | Mean Number of CD44-Positive Epithelial Layers in Leukoplakia (40 × 10) | Mean number of CD9-Positive Epithelial Layers in Leukoplakia (40 × 10) | Macrophages Mean Number, in One Field of View (40 × 10) | SolCD44, Reaction Positive/Negative | Color Intensity of Total Protein (TP) | Grade of Dysplasia in Oral Leukoplakia |
|---|---|---|---|---|---|---|---|---|---|---|
| 1-R | 68 | F | Fundus cavi oris | Homogeneous | 15 | 16 | 23 | - | high 3 | Hyperplasia without dysplasia |
| 2-K | 65 | F | P. lateralis linguae sin | Verrucous | 15 | 13 | 29 | - | high 3 | Hyperplasia without dysplasia |
| 3-P | 59 | F | P. lateralis linguae sin | Nodular | 15 | 28 | 14 | - | high 3 | Mild dysplasia |
| 4-P | 68 | M | P. lateralis linguae dx | Verrucous | 16 | 17 | 11 | - | high 3 | Mild dysplasia |
| 5-E | 27 | M | Muccosa buccae dx | Erythroleukoplakia | 14 | 15 | 15 | - | high 3 | Severe |
| 6-T | 56 | F | P. lateralis linguae dx | Erythroleukoplakia | 15 | 25 | 29 | - | low 2 | Hyperplasia without dysplasia |
| 7-M | 50 | F | Fundus cavi oris | Erythroleukoplakia | 18 | 19 | 23 | + | high 4 | Severe |
| 8-K | 62 | M | Muccosa buccae sin | Nodular | 17 | 17 | 17 | + | high 3 | Mild dysplasia |
| 9-B | 40 | F | Muccosa buccae dx | Erythroleukoplakia | 21 | 20 | 23 | + | low 2 | Mild dysplasia |
| 10-P | 59 | F | Muccosa buccae sin | Homogeneous | 11 | 16 | 28 | + | low 2 | Hyperplasia without dysplasia |
| 11-L | 78 | F | Fundus cavi oris | Erythroleukoplakia | 11 | 18 | 21 | + | high 4 | Severe |
| 12-D | 62 | F | Muccosa buccae sin | Erythroleukoplakia | 16 | 16 | 24 | + | high 3 | Moderate |
| 13-O | 55 | M | Muccosa buccae sin | Verrucous | 15 | 16 | 23 | + | low 2 | Hyperplasia without dysplasia |
| 14-V | 65 | F | Muccosa buccae dx | Erythroleukoplakia | 18 | 19 | 22 | + | low 2 | Hyperplasia without dysplasia |
| 15-P | 38 | M | Fundus cavi oris | Erythroleukoplakia | 13 | 15 | 23 | + | high 3 | Severe |
| 16-S | 63 | F | Muccosa buccae sin | Nodular | 16 | 16 | 25 | - | low 2 | Hyperplasia without dysplasia |
| 17-P | 66 | M | Fundus cavi oris | Verrucous | 19 | 28 | 22 | + | low 1 | Hyperplasia without dysplasia |
| 18-M | 37 | M | Mucosa proc. Alveolaris mandibulae sin | Homogeneous | 19 | 21 | 14 | + | low 2 | Mild dysplasia |
| 19-G | 81 | M | P. lateralis linguae sin | Erythroleukoplakia | 17 | 17 | 17 | + | high 3 | Severe |
| 20-M | 56 | F | Muccosa buccae dx | Homogeneous | 24 | 27 | 24 | - | low 2 | Hyperplasia without dysplasia |
| 21-R | 41 | M | Fundus cavi oris | Homogeneous | 15 | 17 | 28 | - | low 2 | Hyperplasia without dysplasia |
| 22-S | 82 | M | P. lateralis linguae sin | Verrucous | 18 | 18 | 12 | + | high 3 | Mild dysplasia |
| 23-O | 79 | M | Muccosa buccae sin | Erythroleukoplakia | 10 | 9 | 8 | + | high 4 | Ca in situ |
| 24-V | 64 | M | Muccosa labii inf | Verrucous | 10 | 10 | 5 | + | high 3 | Moderate |
| 25-J | 29 | M | Muccosa buccae dx | Verrucous | 14 | 24 | 19 | + | high 3 | Moderate |
| 26-S | 34 | M | Muccosa buccae dx | Homogeneous | 18 | 20 | 17 | - | low 2 | Mild dysplasia |
| 27-F | 70 | F | Mucosa proc. Alveolaris mandibulae dx | Homogeneous | 19 | 20 | 27 | + | low 2 | Hyperplasia without dysplasia |
| 28-K | 39 | M | P. lateralis linguae sin | Erythroleukoplakia | 13 | 16 | 23 | + | high 3 | Severe |
| 29-N | 41 | M | P. lateralis linguaes sin | Erythroleukoplakia | 22 | 24 | 17 | - | low 2 | Severe |
| 30-P | 55 | M | Muccosa buccae dx | Verrucous | 14 | 18 | 19 | + | high 3 | Moderate |
| 31-L | 78 | M | P. lateralis linguae sin | Erythroleukoplakia | 25 | 25 | 24 | + | high 3 | Severe |
| 32-A | 63 | F | P. lateralis linguae sin | Erythroleukoplakia | 17 | 18 | 11 | + | high 3 | Severe |
| 33-S | 51 | M | Muccosa buccae sin | Homogeneous | 22 | 24 | 14 | - | low 2 | Mild dysplasia |
| 34-S | 65 | F | Fundus cavi oris | Erythroleukoplakia | 16 | 16 | 11 | + | high 4 | Severe |
| 35-M | 56 | M | P. lateralis linguae sin | Verrucous | 12 | 13 | 12 | + | high 3 | Moderate |
| 36-S | 64 | M | Muccosa buccae sin | Homogeneous | 16 | 17 | 19 | + | high 3 | Hyperplasia without dysplasia |
| 37-S | 29 | F | P. lateralis linguae sin | Homogeneous | 24 | 25 | 22 | - | low 2 | Hyperplasia without dysplasia |
| 38-M | 62 | M | Fundus cavi oris | Verrucous | 12 | 10 | 31 | + | high 3 | Moderate |
| 39-C | 69 | F | Fundus cavi oris | Erythroleukoplakia | 8 | 8 | 9 | + | high 4 | Ca in situ |
| 40-D | 58 | F | Muccosa buccae dx | Homogeneous | 19 | 20 | 27 | - | low 2 | Hyperplasia without dysplasia |
| 41-D | 42 | F | Muccosa labii inf | Homogeneous | 15 | 16 | 28 | - | low 2 | Hyperplasia without dysplasia |
| 42-K | 43 | M | P. lateralis linguae sin | Verrucous | 10 | 12 | 12 | + | high 4 | Moderate |
| 43-K | 49 | F | P. lateralis linguae dx | Homogeneous | 22 | 23 | 14 | + | low 2 | Mild dysplasia |
| 44-O | 63 | M | Muccosa buccae sin | Homogeneous | 18 | 19 | 13 | - | low 2 | Mild dysplasia |
| 45-D | 67 | M | Fundus cavi oris | Homogeneous | 22 | 22 | 18 | - | low 2 | Mild dysplasia |
| 46-R | 66 | F | P. lateralis linguae dx | Homogeneous | 19 | 20 | 15 | - | low 2 | Mild dysplasia |
| 47-B | 49 | M | Fundus cavi oris | Erythroleukoplakia | 11 | 10 | 6 | + | high 4 | Ca in situ |
| 48-J | 77 | M | P. lateralis linguae dx | Homogeneous | 24 | 25 | 24 | - | low 2 | Hyperplasia without dysplasia |
| 49-M | 55 | M | P. lateralis linguae sin | Nodular | 14 | 15 | 23 | + | high 3 | Moderate |
| 50-L | 55 | M | Muccosa buccae dx | Homogeneous | 22 | 23 | 21 | - | low 2 | Hyperplasia without dysplasia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čēma, I.; Dzudzilo, M.; Kleina, R.; Franckevica, I.; Svirskis, Š. Correlation of Soluble CD44 Expression in Saliva and CD44 Protein in Oral Leukoplakia Tissues. Cancers 2021, 13, 5739. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13225739
Čēma I, Dzudzilo M, Kleina R, Franckevica I, Svirskis Š. Correlation of Soluble CD44 Expression in Saliva and CD44 Protein in Oral Leukoplakia Tissues. Cancers. 2021; 13(22):5739. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13225739
Chicago/Turabian StyleČēma, Ingrīda, Madara Dzudzilo, Regīna Kleina, Ivanda Franckevica, and Šimons Svirskis. 2021. "Correlation of Soluble CD44 Expression in Saliva and CD44 Protein in Oral Leukoplakia Tissues" Cancers 13, no. 22: 5739. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13225739