Nexus between PI3K/AKT and Estrogen Receptor Signaling in Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Biology of ERs
3. PI3K-AKT-mTOR Signaling Axis in Breast Cancer
4. Anti-Estrogen Therapies for Breast Cancer
5. Mechanisms of Resistance to Anti-Estrogens
5.1. Ligand-Independent Activation of ERα
5.2. Interplay between PI3K/AKT and ERα Signaling to Overcome the Effects of Anti-Estrogens
5.3. AKT Influences Genome-Wide Binding of ERα and E2-Mediated Gene Expression
5.4. ERα-Mediated Alternative Splicing and Influence of AKT
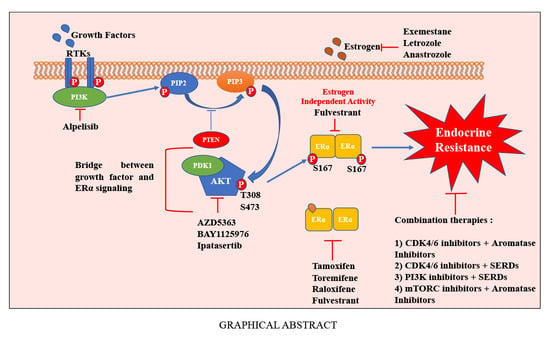
5.5. AKT Is a Bridge between Growth Factor and ERα Signaling
6. Current Clinical Strategies to Treat Anti-Estrogen Resistant Breast Cancers
6.1. Targeting Mutant ERα through New Class of SERDs
6.2. Inhibition of CCND1-CDK4/6-RB Pathway
6.3. Inhibition of PI3K-AKT-mTOR Pathway
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Mph, K.D.M.; Sauer, A.G.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Coombes, R.C. Endocrine-responsive breast cancer and strategies for combating resistance. Nat. Rev. Cancer 2002, 2, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schütz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, S.; Gustafsson, J.-Å. Estrogen Receptors: Therapies Targeted to Receptor Subtypes. Clin. Pharmacol. Ther. 2011, 89, 44–55. [Google Scholar] [CrossRef]
- Welboren, W.-J.; Stunnenberg, H.G.; Sweep, F.C.; Span, P.N. Identifying estrogen receptor target genes. Mol. Oncol. 2007, 1, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, S.; Mäkelä, S.; Treuter, E.; Tujague, M.; Thomsen, J.; Andersson, G.; Enmark, E.; Pettersson, K.; Warner, M.; Gustafsson, J.Å. Mechanisms of Estrogen Action. Physiol. Rev. 2001, 81, 1535–1565. [Google Scholar] [CrossRef]
- Walker, V.R.; Korach, K.S. Estrogen Receptor Knockout Mice as a Model for Endocrine Research. ILAR J. 2004, 45, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Peng, Y.; Kiselar, J.; Zhao, X.; Albaqami, A.; Mendez, D.; Chen, Y.; Chakravarthy, S.; Gupta, S.; Ralston, C.; et al. Multidomain architecture of estrogen receptor reveals interfacial cross-talk between its DNA-binding and ligand-binding domains. Nat. Commun. 2018, 9, 3520. [Google Scholar] [CrossRef]
- Légaré, S.; Basik, M. Minireview: The Link Between ERα Corepressors and Histone Deacetylases in Tamoxifen Resistance in Breast Cancer. Mol. Endocrinol. 2016, 30, 965–976. [Google Scholar] [CrossRef] [Green Version]
- Lonard, D.M.; O’Malley, B.W. Molecular Pathways: Targeting Steroid Receptor Coactivators in Cancer. Clin. Cancer Res. 2016, 22, 5403–5407. [Google Scholar] [CrossRef] [Green Version]
- Jozwik, K.M.; Carroll, J.S. Pioneer factors in hormone-dependent cancers. Nat. Rev. Cancer 2012, 12, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Bhat-Nakshatri, P.; Chu, X.; Liu, Y.; Wang, Y.; Nakshatri, H. Nonlinear relationship between chromatin acces-sility and estradiol-regulated gene expression. Oncogene 2020. [Google Scholar] [CrossRef]
- Siersbæk, R.; Kumar, S.; Carroll, J.S. Signaling pathways and steroid receptors modulating estrogen receptor α function in breast cancer. Genes Dev. 2018, 32, 1141–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folkerd, E.J.; Dowsett, M. Influence of Sex Hormones on Cancer Progression. J. Clin. Oncol. 2010, 28, 4038–4044. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Zhang, H.; Kong, Q.; Jiang, Y. Mechanisms for estrogen receptor expression in human cancer. Exp. Hematol. Oncol. 2018, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Puglisi, R.; Mattia, G.; Carè, A.; Marano, G.; Malorni, W.; Matarrese, P. Non-genomic Effects of Estrogen on Cell Homeostasis and Remodeling With Special Focus on Cardiac Ischemia/Reperfusion Injury. Front. Endocrinol. 2019, 10, 733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalano, S.; Barone, I.; Giordano, C.; Rizza, P.; Qi, H.; Gu, G.; Malivindi, R.; Bonofiglio, D.; Andò, S. Rapid Estradiol/ERα Signaling Enhances Aromatase Enzymatic Activity in Breast Cancer Cells. Mol. Endocrinol. 2009, 23, 1634–1645. [Google Scholar] [CrossRef] [Green Version]
- Pedram, A.; Razandi, M.; Levin, E.R. Nature of Functional Estrogen Receptors at the Plasma Membrane. Mol. Endocrinol. 2006, 20, 1996–2009. [Google Scholar] [CrossRef]
- Resh, M.D. Fatty acylation of proteins: New insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta (BBA) Bioenerg. 1999, 1451, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Chambliss, K.L.; Shaul, P.W. Estrogen Modulation of Endothelial Nitric Oxide Synthase. Endocr. Rev. 2002, 23, 665–686. [Google Scholar] [CrossRef] [Green Version]
- Razandi, M.; Pedram, A.; Merchenthaler, I.; Greene, G.L.; Levin, E.R. Plasma Membrane Estrogen Receptors Exist and Functions as Dimers. Mol. Endocrinol. 2004, 18, 2854–2865. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-R.; Park, J.; Yu, H.-N.; Kim, J.-S.; Youn, H.J.; Jung, S.H. Up-regulation of PI3K/Akt signaling by 17β-estradiol through activation of estrogen receptor-α, but not estrogen receptor-β, and stimulates cell growth in breast cancer cells. Biochem. Biophys. Res. Commun. 2005, 336, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M.; Blankenship, K.A.; Risinger, K.E.; Bhatnagar, S.; Noisin, E.L.; Sumanasekera, W.K.; Zhao, L.; Brey, D.M.; Keynton, R.S. Resveratrol and Estradiol Rapidly Activate MAPK Signaling through Estrogen Receptors α and β in Endothelial Cells. J. Biol. Chem. 2005, 280, 7460–7468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zivadinovic, D.; Watson, C.S. Membrane estrogen receptor-α levels predict estrogen-induced ERK1/2 activation in MCF-7 cells. Breast Cancer Res. 2004, 7, R130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santen, R.; Jeng, M.-H.; Wang, J.-P.; Song, R.; Masamura, S.; McPherson, R.; Santner, S.; Yue, W.; Shim, W.-S. Adaptive hypersensitivity to estradiol: Potential mechanism for secondary hormonal responses in breast cancer patients. J. Steroid Biochem. Mol. Biol. 2001, 79, 115–125. [Google Scholar] [CrossRef]
- Revathidevi, S.; Arasambattu, M. Akt in cancer: Mediator and more. Semin. Cancer Biol. 2019, 59, 80–91. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB Signaling: Navigating Downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef] [Green Version]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [Green Version]
- Suyama, K.; Yao, J.; Liang, H.; Benard, O.; Loudig, O.; Amgalan, D.; McKimpson, W.M.; Phillips, G.R.; Segall, J.; Wang, Y.; et al. An Akt3 Splice Variant Lacking the Serine 472 Phosphorylation Site Promotes Apoptosis and Suppresses Mammary Tumorigenesis. Cancer Res. 2018, 78, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Ebner, M.; Lučić, I.; Leonard, T.A.; Yudushkin, I. PI(3,4,5)P 3 Engagement Restricts Akt Activity to Cellular Membranes. Mol. Cell 2017, 65, 416–431.e6. [Google Scholar] [CrossRef] [Green Version]
- Lučić, I.; Rathinaswamy, M.K.; Truebestein, L.; Hamelin, D.J.; Burke, J.E.; Leonard, T.A. Conformational sampling of membranes by Akt controls its activation and inactivation. Proc. Natl. Acad. Sci. USA 2018, 115, E3940–E3949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facchinetti, V.; Ouyang, W.; Wei, H.; Soto, N.; Lazorchak, A.; Gould, C.; Lowry, C.; Newton, A.C.; Mao, Y.; Miao, R.Q.; et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 2008, 27, 1932–1943. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, P.P.; Chiu, K.E.; François, F.M.; Scott, J.L.; Khorjekar, G.R.; Tirada, N.P. Genetic Testing and Screening Recommendations for Patients with Hereditary Breast Cancer. RadioGraphics 2020, 40, 913–936. [Google Scholar] [CrossRef] [PubMed]
- Coticchia, C.M.; Revankar, C.M.; Deb, T.B.; Dickson, R.B.; Johnson, M.D. Calmodulin modulates Akt activity in human breast cancer cell lines. Breast Cancer Res. Treat. 2008, 115, 545–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klinghoffer, R.A.; Duckworth, B.; Valius, M.; Cantley, L.C.; Kazlauskas, A. Platelet-derived growth factor-dependent activation of phosphatidylinositol 3-kinase is regulated by receptor binding of SH2-domain-containing proteins which influence Ras activity. Mol. Cell. Biol. 1996, 16, 5905–5914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wollmann, W.; Goodman, M.L.; Bhat-Nakshatri, P.; Kishimoto, H.; Goulet, R.J.; Mehrotra, S.; Morimiya, A.; Badve, S.; Nakshatri, H. The macrophage inhibitory cytokine integrates AKT/PKB and MAP kinase signaling pathways in breast cancer cells. Carcinogenesis 2005, 26, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Hinz, N.; Jücker, M. Distinct functions of AKT isoforms in breast cancer: A comprehensive review. Cell Commun. Signal. 2019, 17, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Maroulakou, I.G.; Oemler, W.; Naber, S.P.; Tsichlis, P.N. Akt1 Ablation Inhibits, whereas Akt2 Ablation Accelerates, the Development of Mammary Adenocarcinomas in Mouse Mammary Tumor Virus (MMTV)-ErbB2/Neu and MMTV-Polyoma Middle T Transgenic Mice. Cancer Res. 2007, 67, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Liu, Z.; Xu, H.; Yang, Q. miR-409-3p suppresses breast cancer cell growth and invasion by targeting Akt1. Biochem. Biophys. Res. Commun. 2016, 469, 189–195. [Google Scholar] [CrossRef]
- Bozulic, L.; Surucu, B.; Hynx, D.; Hemmings, B.A. PKBα/Akt1 Acts Downstream of DNA-PK in the DNA Double-Strand Break Response and Promotes Survival. Mol. Cell 2008, 30, 203–213. [Google Scholar] [CrossRef]
- Choi, J.-A.; Jung, Y.S.; Kim, J.Y.; Kim, H.M.; Lim, I.K. Inhibition of breast cancer invasion by TIS21/BTG2/Pc3-Akt1-Sp1-Nox4 pathway targeting actin nucleators, mDia genes. Oncogene 2016, 35, 83–93. [Google Scholar] [CrossRef]
- Xu, H.; Lin, F.; Wang, Z.; Yang, L.; Meng, J.; Ou, Z.; Shao, Z.-M.; Di, G.; Yang, G. CXCR2 promotes breast cancer metastasis and chemoresistance via suppression of AKT1 and activation of COX2. Cancer Lett. 2018, 412, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Badve, S.; Collins, N.R.; Bhat-Nakshatri, P.; Turbin, D.; Leung, S.; Thorat, M.A.; Dunn, S.E.; Geistlinger, T.R.; Carroll, J.S.; Brown, M.; et al. Subcellular Localization of Activated AKT in Estrogen Receptor- and Progesterone Receptor-Expressing Breast Cancers. Am. J. Pathol. 2010, 176, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Attoub, S.; Arafat, K.; Hammadi, N.K.; Mester, J.; Gaben, A.-M. Akt2 knock-down reveals its contribution to human lung cancer cell proliferation, growth, motility, invasion and endothelial cell tube formation. Sci. Rep. 2015, 5, 12759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, Y.R.; Yuan, X.; Balk, S.P.; Toker, A. PTEN-Deficient Tumors Depend on AKT2 for Maintenance and Survival. Cancer Discov. 2014, 4, 942–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arboleda, M.J.; Lyons, J.F.; Kabbinavar, F.F.; Bray, M.R.; Snow, B.E.; Ayala, R.; Danino, M.; Karlan, B.Y.; Slamon, D.J. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 2003, 63, 196–206. [Google Scholar] [PubMed]
- Chin, Y.R.; Toker, A. The Actin-Bundling Protein Palladin Is an Akt1-Specific Substrate that Regulates Breast Cancer Cell Migration. Mol. Cell 2010, 38, 333–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabinski, N.; Möllmann, K.; Milde-Langosch, K.; Müller, V.; Schumacher, U.; Brandt, B.; Pantel, K.; Jücker, M. AKT3 regulates ErbB2, ErbB3 and estrogen receptor α expression and contributes to endocrine therapy resistance of ErbB2+ breast tumor cells from Balb-neuT mice. Cell. Signal. 2014, 26, 1021–1029. [Google Scholar] [CrossRef]
- Banerji, S.; Cibulskis, K.; Rangel-Escareno, C.; Brown, K.K.; Carter, S.L.; Frederick, A.M.; Lawrence, M.S.; Sivachenko, A.Y.; Sougnez, C.; Zou, L.; et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nat. Cell Biol. 2012, 486, 405–409. [Google Scholar] [CrossRef]
- Chin, Y.R.; Yoshida, T.; Marusyk, A.; Beck, A.H.; Polyak, K.; Toker, A. Targeting Akt3 signaling in triple-negative breast cancer. Cancer Res. 2014, 74, 964–973. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Cai, B.; Shen, L.; Dong, Y.; Lu, Q.; Sun, S.; Liu, S.; Ma, S.; Ma, P.X.; Chen, J.-H. MiRNA-29b suppresses tumor growth through simultaneously inhibiting angiogenesis and tumorigenesis by targeting Akt3. Cancer Lett. 2017, 397, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data: Figure 1. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparano, J.A.; Gray, R.J.; Ravdin, P.M.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E.; Dees, E.C.; Goetz, M.P.; et al. Clinical and Genomic Risk to Guide the Use of Adjuvant Therapy for Breast Cancer. N. Engl. J. Med. 2019, 380, 2395–2405. [Google Scholar] [CrossRef] [PubMed]
- Najim, O.; Seghers, S.; Sergoynne, L.; Van Gaver, H.; Papadimitriou, K.; Wouters, K.; Trinh, X.B.; Huizing, M.T.; Tjalma, W. The association between type of endocrine therapy and development of estrogen receptor-1 mutation(s) in patients with hormone-sensitive advanced breast cancer: A systematic review and meta-analysis of randomized and non-randomized trials. Biochim. Biophys. Acta (BBA) Bioenerg. 2019, 1872, 188315. [Google Scholar] [CrossRef]
- Almeida, C.F.; Oliveira, A.; Ramos, M.J.; Fernandes, P.A.; Teixeira, N.; Amaral, C. Estrogen receptor-positive (ER+) breast cancer treatment: Are multi-target compounds the next promising approach? Biochem. Pharmacol. 2020, 177, 113989. [Google Scholar] [CrossRef]
- Merenbakh-Lamin, K.; Ben-Baruch, N.; Yeheskel, A.; Dvir, A.; Soussan-Gutman, L.; Jeselsohn, R.; Yelensky, R.; Brown, M.; Miller, V.A.; Sarid, D.; et al. D538G Mutation in Estrogen Receptor-α: A Novel Mechanism for Acquired Endocrine Resistance in Breast Cancer. Cancer Res. 2013, 73, 6856–6864. [Google Scholar] [CrossRef] [Green Version]
- Saha, T.; Makar, S.; Swetha, R.; Gutti, G.; Singh, S.K. Estrogen signaling: An emanating therapeutic target for breast cancer treatment. Eur. J. Med. Chem. 2019, 177, 116–143. [Google Scholar] [CrossRef]
- Pinkerton, J.V.; Thomas, S. Use of SERMs for treatment in postmenopausal women. J. Steroid Biochem. Mol. Biol. 2014, 142, 142–154. [Google Scholar] [CrossRef]
- D’Amelio, P.; Isaia, G.C. The use of raloxifenein osteoporosis treatment. Expert Opin. Pharmacother. 2013, 14, 949–956. [Google Scholar] [CrossRef]
- Kumar, P.; Song, Z.-H. CB2 cannabinoid receptor is a novel target for third-generation selective estrogen receptor modulators bazedoxifene and lasofoxifene. Biochem. Biophys. Res. Commun. 2014, 443, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Cummings, S.R.; McClung, M.; Reginster, J.-Y.; Cox, D.; Mitlak, B.; Stock, J.; Amewou-Atisso, M.; Powles, T.; Miller, P.; Zanchetta, J.; et al. Arzoxifene for prevention of fractures and invasive breast cancer in postmenopausal women. J. Bone Miner. Res. 2011, 26, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.K.; Bihani, T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol. Ther. 2018, 186, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Wardell, S.E.; Nelson, E.R.; Chao, C.A.; Alley, H.M.; McDonnell, D.P. Evaluation of the pharmacological activities of RAD1901, a selective estrogen receptor degrader. Endocr. Relat. Cancer 2015, 22, 713–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlson, R.W. The History and Mechanism of Action of Fulvestrant. Clin. Breast Cancer 2005, 6, S5–S8. [Google Scholar] [CrossRef]
- Soleja, M.; Raj, G.V.; Unni, N. An evaluation of fulvestrant for the treatment of metastatic breast cancer. Expert Opin. Pharmacother. 2019, 20, 1819–1829. [Google Scholar] [CrossRef]
- Gombos, A. Selective oestrogen receptor degraders in breast cancer. Curr. Opin. Oncol. 2019, 31, 424–429. [Google Scholar] [CrossRef]
- Bardia, A.; Aftimos, P.; Bihani, T.; Anderson-Villaluz, A.T.; Jung, J.; Conlan, M.G.; Kaklamani, V.G. EMERALD: Phase III trial of elacestrant (RAD1901) vs endocrine therapy for previously treated ER+ advanced breast cancer. Future Oncol. 2019, 15, 3209–3218. [Google Scholar] [CrossRef]
- Hamilton, E.; Patel, M.R.; Armstrong, A.C.; Baird, R.D.; Jhaveri, K.; Hoch, M.; Klinowska, T.; Lindemann, J.P.; Morgan, S.R.; Schiavon, G.; et al. A First-in-Human Study of the New Oral Selective Estrogen Receptor Degrader AZD9496 for ER+/HER2− Advanced Breast Cancer. Clin. Cancer Res. 2018, 24, 3510–3518. [Google Scholar] [CrossRef] [Green Version]
- Dickler, M.N.; Villanueva, R.; Fidalgo, J.A.P.; Mayer, I.A.; Boni, V.; Winer, E.P. A first-in-human phase i study to evaluate the oral estrogen receptor degrader (serd), gdc-0927, in postmenopausal women with estrgeon receptor positive (er+) her2-negative metastatic breast cancer. Cancer Res. 2017, 78, PD5–PD10. [Google Scholar]
- Jhaveri, K.; Juric, D.; Yap, Y.; Cresta, S.; Layman, R.M.; Duhoux, F.P.; Terret, C.; de Vita, S.; Kundamal, N.; He, W.; et al. Interim results of a phase i/ib study of lsz102, an oral selective estrogen receptor degrad-er (serd), in combination with ribociclib or alpelisib in patients with er+ breast cancer who had prgoressed after endocrine therapy. Ann. Oncol. 2020, 31, S62–S82. [Google Scholar] [CrossRef]
- Chumsri, S.; Howes, T.; Bao, T.; Sabnis, G.; Brodie, A. Aromatase, aromatase inhibitors, and breast cancer. J. Steroid Biochem. Mol. Biol. 2011, 125, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Kümler, I.; Knoop, A.S.; Jessing, C.A.; Ejlertsen, B.; Nielsen, D.L. Review of hormone-based treatments in postmenopausal patients with advanced breast cancer focusing on aromatase inhibitors and fulvestrant. ESMO Open 2016, 1, e000062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wang, Z.; Shao, Z. Fulvestrant in the treatment of hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: A review. Cancer Med. 2019, 8, 1943–1957. [Google Scholar] [CrossRef] [PubMed]
- Nathan, M.R.; Schmid, P. A Review of Fulvestrant in Breast Cancer. Oncol. Ther. 2017, 5, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Toy, W.; Weir, H.; Razavi, P.; Lawson, M.; Goeppert, A.U.; Mazzola, A.M.; Smith, A.; Wilson, J.; Morrow, C.; Wong, W.L.; et al. Activating ESR1 Mutations Differentially Affect the Efficacy of ER Antagonists. Cancer Discov. 2017, 7, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Santarpia, L.; Bottai, G.; Kelly, C.M.; Győrffy, B.; Székely, B.; Pusztai, L. Deciphering and Targeting Oncogenic Mutations and Pathways in Breast Cancer. Oncololgy 2016, 21, 1063–1078. [Google Scholar] [CrossRef] [Green Version]
- Cheung, K.W.K.; Yoshida, K.; Cheeti, S.; Chen, B.; Morley, R.; Chan, I.T.; Sahasranaman, S.; Liu, L. GDC-0810 Pharmacokinetics and Transporter-Mediated Drug Interaction Evaluation with an Endogenous Biomarker in the First-in-Human, Dose Escalation Study. Drug Metab. Dispos. 2019, 47, 966–973. [Google Scholar] [CrossRef]
- Beaver, J.A.; Amiri-Kordestani, L.; Charlab, R.; Chen, W.; Palmby, T.; Tilley, A.; Zirkelbach, J.F.; Yu, J.; Liu, Q.; Zhao, L.; et al. FDA Approval: Palbociclib for the Treatment of Postmenopausal Patients with Estrogen Receptor–Positive, HER2-Negative Metastatic Breast Cancer. Clin. Cancer Res. 2015, 21, 4760–4766. [Google Scholar] [CrossRef] [Green Version]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Walker, A.J.; Wedam, S.; Amiri-Kordestani, L.; Bloomquist, E.; Tang, S.; Sridhara, R.; Chen, W.; Palmby, T.R.; Zirkelbach, J.F.; Fu, W.; et al. FDA Approval of Palbociclib in Combination with Fulvestrant for the Treatment of Hormone Receptor–Positive, HER2-Negative Metastatic Breast Cancer. Clin. Cancer Res. 2016, 22, 4968–4972. [Google Scholar] [CrossRef] [Green Version]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickler, M.N.; Tolaney, S.M.; Rugo, H.S.; Cortés, J.; Diéras, V.; Patt, D.; Wildiers, H.; Hudis, C.A.; O’Shaughnessy, J.; Zamora, E.; et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR+/HER2− Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 5218–5224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sledge, G.W., Jr.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2− Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.-C.; Manso, L.; et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef] [PubMed]
- Im, S.-A.; Lu, Y.-S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.-S.; Campos-Gomez, S.; et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019, 381, 307–316. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Beaver, J.A.; Park, B.H. The BOLERO-2 trial: The addition of everolimus to exemestane in the treatment of postmenopausal hormone receptor-positive advanced breast cancer. Future Oncol. 2012, 8, 651–657. [Google Scholar] [CrossRef] [Green Version]
- Baselga, J.; Campone, M.; Piccart-Gebhart, M.; Burris, H.A.; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in Postmenopausal Hormone-Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef] [Green Version]
- Wander, S.A.D.J.; Supko, J.G.; Micalizzi, D.S.; Spring, L.; Vidula, N.; Beeler, M.; Habin, K.R.; Viscosi, E. Phase ib trial to evaluate safety and anti-tumor activity of the akt inhibitor, ipatasertib, in combination with endocrine therapy and a cdk4/6 inhibitor for patients with hormone receptor positive (hr+)/her2 negative metastatic breast cancer (taktic). J. Clin. Oncol. 2020, 38, 1066-1066. [Google Scholar] [CrossRef]
- Pascual, J.; Lim, J.S.; MacPherson, I.R.J.; Armstrong, A.C.; Ring, A.; Okines, A.F.; Cutts, R.J.; Herrera-Abreu, M.T.; Garcia-Murillas, I.; Pearson, A.; et al. Triplet Therapy with Palbociclib, Taselisib, and Fulvestrant in PIK3CA-Mutant Breast Cancer and Doublet Palbociclib and Taselisib in Pathway-Mutant Solid Cancers. Cancer Discov. 2020, 11, 92–107. [Google Scholar] [CrossRef]
- Brier, M.J.; Chambless, D.L.; Gross, R.; Chen, J.; Mao, J.J. Perceived barriers to treatment predict adherence to aromatase inhibitors among breast cancer survivors. Cancer 2016, 123, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Goss, P.E.; Strasser, K. Aromatase Inhibitors in the Treatment and Prevention of Breast Cancer. J. Clin. Oncol. 2001, 19, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Augusto, T.V.; Correia-Da-Silva, G.; Rodrigues, C.M.; Teixeira, N.; Amaral, C. Acquired resistance to aromatase inhibitors: Where we stand! Endocr. Related Cancer 2018, 25, R283–R301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sluyser, M.; Mester, J. Oncogenes homologous to steroid receptors? Nat. Cell Biol. 1985, 315, 546. [Google Scholar] [CrossRef]
- Karnik, P.S.; Kulkarni, S.; Liu, X.P.; Budd, G.T.; Bukowski, R.M. Estrogen receptor mutations in tamoxifen-resistant breast cancer. Cancer Res. 1994, 54, 349–353. [Google Scholar]
- Inoue, A.; Yoshida, N.; Omoto, Y.; Oguchi, S.; Yamori, T.; Kiyama, R.; Hayashi, S. Development of cDNA microarray for expression profiling of estrogen-responsive genes. J. Mol. Endocrinol. 2002, 29, 175–192. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.X.; Borg, A.; Wolf, D.M.; Oesterreich, S.; Fuqua, S.A. An estrogen receptor mutant with strong hor-mone-independent activity from a metastatic breast cancer. Cancer Res. 1997, 57, 1244–1249. [Google Scholar]
- Robinson, D.R.; Wu, Y.-M.; Vats, P.; Su, F.; Lonigro, R.J.; Cao, X.; Kalyana-Sundaram, S.; Wang, R.; Ning, Y.; Hodges, L.; et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat. Genet. 2013, 45, 1446–1451. [Google Scholar] [CrossRef] [Green Version]
- Griffith, O.L.; Spies, N.C.; Anurag, M.; Griffith, M.; Luo, J.; Tu, D.; Yeo, B.; Kunisaki, J.; Miller, C.A.; Krysiak, K.; et al. The prognostic effects of somatic mutations in ER-positive breast cancer. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- De Leeuw, R.; Neefjes, J.; Michalides, R. A Role for Estrogen Receptor Phosphorylation in the Resistance to Tamoxifen. Int. J. Breast Cancer 2011, 2011, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Le Romancer, M.; Treilleux, I.; Leconte, N.; Robin-Lespinasse, Y.; Sentis, S.; Bouchekioua-Bouzaghou, K.; Goddard, S.; Gobert-Gosse, S.; Corbo, L. Regulation of Estrogen Rapid Signaling through Arginine Methylation by PRMT1. Mol. Cell 2008, 31, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Woo, E.M.; Chong, Y.T.E.; Homenko, D.R.; Kraus, W.L. Acetylation of Estrogen Receptor α by p300 at Lysines 266 and 268 Enhances the Deoxyribonucleic Acid Binding and Transactivation Activities of the Receptor. Mol. Endocrinol. 2006, 20, 1479–1493. [Google Scholar] [CrossRef] [PubMed]
- Le Romancer, M.; Poulard, C.; Cohen, P.; Sentis, S.; Renoir, J.-M.; Corbo, L. Cracking the Estrogen Receptor’s Posttranslational Code in Breast Tumors. Endocr. Rev. 2011, 32, 597–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondakova, I.V.; Shashova, E.E.; Sidenko, E.A.; Astakhova, T.M.; Zakharova, L.; Sharova, N.P. Estrogen Receptors and Ubiquitin Proteasome System: Mutual Regulation. Biomolecules 2020, 10, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajbhandari, P.; Schalper, K.A.; Solodin, N.M.; Ellison-Zelski, S.J.; Lu, K.P.; Rimm, D.L.; Alarid, E.T. Pin1 modulates ERα levels in breast cancer through inhibition of phosphorylation-dependent ubiquitination and degradation. Oncogene 2013, 33, 1438–1447. [Google Scholar] [CrossRef] [Green Version]
- Busonero, C.; Leone, S.; Bianchi, F.; Maspero, E.; Fiocchetti, M.; Palumbo, O.; Cipolletti, M.; Bartoloni, S.; Acconcia, F. Ouabain and Digoxin Activate the Proteasome and the Degradation of the ERα in Cells Modeling Primary and Metastatic Breast Cancer. Cancers 2020, 12, 3840. [Google Scholar] [CrossRef]
- Campbell, R.A.; Bhat-Nakshatri, P.; Patel, N.M.; Constantinidou, D.; Ali, S.; Nakshatri, H. Phosphatidylinositol 3-Kinase/AKT-mediated Activation of Estrogen Receptor α. J. Biol. Chem. 2001, 276, 9817–9824. [Google Scholar] [CrossRef] [Green Version]
- Bostner, J.; Karlsson, E.; Pandiyan, M.J.; Westman, H.; Skoog, L.; Fornander, T.; Nordenskjöld, B.; Stål, O. Activation of Akt, mTOR, and the estrogen receptor as a signature to predict tamoxifen treatment benefit. Breast Cancer Res. Treat. 2012, 137, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Hasson, S.P.; Rubinek, T.; Ryvo, L.; Wolf, I. Endocrine Resistance in Breast Cancer: Focus on the Phosphatidylinositol 3-Kinase/Akt/Mammalian Target of Rapamycin Signaling Pathway. Breast Care 2013, 8, 248–255. [Google Scholar] [CrossRef] [Green Version]
- Carroll, J.S.; Liu, X.S.; Brodsky, A.S.; Li, W.; Meyer, C.A.; Szary, A.J.; Eeckhoute, J.; Shao, W.; Hestermann, E.V.; Geistlinger, T.R.; et al. Chromosome-Wide Mapping of Estrogen Receptor Binding Reveals Long-Range Regulation Requiring the Forkhead Protein FoxA1. Cell 2005, 122, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Carroll, J.S.; Meyer, C.A.; Song, J.; Li, W.; Geistlinger, T.R.; Eeckhoute, J.; Brodsky, A.S.; Keeton, E.K.; Fertuck, K.C.; Hall, G.F.; et al. Genome-wide analysis of estrogen receptor binding sites. Nat. Genet. 2006, 38, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Bhat-Nakshatri, P.; Wang, G.; Appaiah, H.; Luktuke, N.; Carroll, J.S.; Geistlinger, T.R.; Brown, M.; Badve, S.; Liu, Y.; Nakshatri, H. AKT Alters Genome-Wide Estrogen Receptor α Binding and Impacts Estrogen Signaling in Breast Cancer. Mol. Cell. Biol. 2008, 28, 7487–7503. [Google Scholar] [CrossRef] [Green Version]
- Bhat-Nakshatri, P.; Wang, G.; Collins, N.R.; Thomson, M.J.; Geistlinger, T.R.; Carroll, J.S.; Brown, M.; Hammond, S.; Srour, E.F.; Liu, Y.; et al. Estradiol-regulated microRNAs control estradiol response in breast cancer cells. Nucleic Acids Res. 2009, 37, 4850–4861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat-Nakshatri, P.; Goswami, C.P.; Badve, S.; Magnani, L.; Lupien, M.; Nakshatri, H. Molecular Insights of Pathways Resulting from Two Common PIK3CA Mutations in Breast Cancer. Cancer Res. 2016, 76, 3989–4001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toska, E.; Osmanbeyoglu, H.U.; Castel, P.; Chan, C.; Hendrickson, R.C.; Elkabets, M.; Dickler, M.N.; Scaltriti, M.; Leslie, C.; Armstrong, S.A.; et al. PI3K pathway regulates ER-dependent transcription in breast cancer through the epigenetic regulator KMT2D. Science 2017, 355, 1324–1330. [Google Scholar] [CrossRef] [Green Version]
- Toska, E.; Castel, P.; Chhangawala, S.; Arruabarrena-Aristorena, A.; Chan, C.; Hristidis, V.C.; Cocco, E.; Sallaku, M.; Xu, G.; Park, J.; et al. PI3K Inhibition Activates SGK1 via a Feedback Loop to Promote Chromatin-Based Regulation of ER-Dependent Gene Expression. Cell Rep. 2019, 27, 294–306.e5. [Google Scholar] [CrossRef] [Green Version]
- Schwerk, C.; Schulze-Osthoff, K. Regulation of apoptosis by alternative pre-mrna splicing. Mol. Cell 2005, 19, 1–13. [Google Scholar] [CrossRef]
- Pritsker, M.; Doniger, T.T.; Kramer, L.C.; Westcot, S.E.; Lemischka, I.R. Diversification of stem cell molecular reper-toire by alternative splicing. Proc. Natl. Acad. Sci. USA 2005, 102, 14290–14295. [Google Scholar] [CrossRef] [Green Version]
- Dutertre, M.; Vagner, S.; Auboeuf, D. Alternative splicing and breast cancer. RNA Biol. 2010, 7, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Grammatopoulos, D.K. CRH-R splicing in estrogen-sensitive breast cancer. Cell Cycle 2014, 13, 687–688. [Google Scholar] [CrossRef] [Green Version]
- Bhat-Nakshatri, P.; Song, E.-K.; Collins, N.R.; Uversky, V.N.; Dunker, A.K.; O’Malley, B.W.; Geistlinger, T.R.; Carroll, J.S.; Brown, M.; Nakshatri, H. Interplay between estrogen receptor and AKT in Estradiol-induced alternative splicing. BMC Med. Genom. 2013, 6, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turczyk, L.; Kitowska, K.; Mieszkowska, M.; Mieczkowski, K.; Czaplinska, D.; Piasecka, D.; Kordek, R.; Skladanowski, A.C.; Potemski, P.; Romanska, H.M.; et al. FGFR2-Driven Signaling Counteracts Tamoxifen Effect on ERα-Positive Breast Cancer Cells. Neoplasia 2017, 19, 791–804. [Google Scholar] [CrossRef]
- Osborne, C.K.; Schiff, R. Mechanisms of Endocrine Resistance in Breast Cancer. Annu. Rev. Med. 2011, 62, 233–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, T.W.; Balko, J.M.; Arteaga, C.L. Phosphatidylinositol 3-Kinase and Antiestrogen Resistance in Breast Cancer. J. Clin. Oncol. 2011, 29, 4452–4461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, T.W.; Rexer, B.N.; Garrett, J.T.; Arteaga, C.L. Mutations in the phosphatidylinositol 3-kinase pathway: Role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 2011, 13, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudu, V.; Able, R.A.; Rotari, V.; Kong, Q.; Vazquez, M. Role of Epidermal Growth Factor-Triggered PI3K/Akt Signaling in the Migration of Medulloblastoma-Derived Cells. Cell. Mol. Bioeng. 2012, 5, 402–413. [Google Scholar] [CrossRef] [Green Version]
- Gross, S.M.; Rotwein, P. Mapping growth-factor-modulated Akt signaling dynamics. J. Cell Sci. 2016, 129, 2052–2063. [Google Scholar] [CrossRef] [Green Version]
- Lupien, M.; Meyer, C.A.; Bailey, S.T.; Eeckhoute, J.; Cook, J.; Westerling, T.; Zhang, X.; Carroll, J.S.; Rhodes, D.R.; Liu, X.S.; et al. Growth factor stimulation induces a distinct ER cistrome underlying breast cancer endocrine resistance. Genes Dev. 2010, 24, 2219–2227. [Google Scholar] [CrossRef] [Green Version]
- Frogne, T.; Jepsen, J.S.; Larsen, S.S.; Fog, C.; Brockdorff, B.L.; Lykkesfeldt, A.E. Antiestrogen-resistant human breast cancer cells require activated Protein Kinase B/Akt for growth. Endocr. Relat. Cancer 2005, 12, 599–614. [Google Scholar] [CrossRef] [Green Version]
- Creighton, C.J.; Fu, X.; Hennessy, B.T.; Casa, A.J.; Zhang, Y.; Gonzalez-Angulo, A.M.; Lluch, A.; Gray, J.W.; Brown, P.H.; Hilsenbeck, S.G.; et al. Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res. 2010, 12, R40. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Zhang, X.; Pintilie, M.; Ma, N.; Miller, N.; Banerjee, D.; Tsao, M.-S.; Mak, T.; Fyles, A.; Liu, F.-F. Dysregulated PTEN-PKB and negative receptor status in human breast cancer. Int. J. Cancer 2003, 104, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.W.; Hennessy, B.T.; González-Angulo, A.M.; Fox, E.M.; Mills, G.B.; Chen, H.; Higham, C.; García-Echeverría, C.; Shyr, Y.; Arteaga, C.L. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor–positive human breast cancer. J. Clin. Investig. 2010, 120, 2406–2413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carracedo, A.; Ma, L.; Teruya-Feldstein, J.; Rojo, F.; Salmena, L.; Alimonti, A.; Egia, A.; Sasaki, A.T.; Thomas, G.; Kozma, S.C.; et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J. Clin. Investig. 2008, 118, 3065–3074. [Google Scholar] [CrossRef]
- Chandarlapaty, S.; Sawai, A.; Scaltriti, M.; Rodrik-Outmezguine, V.; Grbovic-Huezo, O.; Serra, V.; Majumder, P.K.; Baselga, J.; Rosen, N. AKT Inhibition Relieves Feedback Suppression of Receptor Tyrosine Kinase Expression and Activity. Cancer Cell 2011, 19, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Schiavon, G.; Hrebien, S.; Garcia-Murillas, I.; Cutts, R.J.; Pearson, A.; Tarazona, N.; Fenwick, K.; Kozarewa, I.; Lopez-Knowles, E.; Ribas, R.; et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci. Transl. Med. 2015, 7, 313ra182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toy, W.; Shen, Y.; Won, H.; Green, B.; Sakr, R.A.; Will, M.; Li, Z.; Gala, K.; Fanning, S.; King, T.A.; et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat. Genet. 2013, 45, 1439–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Fernández, M.; Malumbres, M. Mechanisms of Sensitivity and Resistance to CDK4/6 Inhibition. Cancer Cell 2020, 37, 514–529. [Google Scholar] [CrossRef]
- Pandey, K.; An, H.; Kim, S.K.; Lee, S.A.; Kim, S.; Lim, S.M.; Kim, G.M.; Sohn, J.; Moon, Y.W. Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: A review. Int. J. Cancer 2019, 145, 1179–1188. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, A.; Lindström, L.S.; Li, J.; Harrell, J.C.; Darai-Ramqvist, E.; Sifakis, E.; Foukakis, T.; Perou, C.M.; Czene, K.; Bergh, J.; et al. The long-term prognostic and predictive capacity of cyclin D1 gene amplification in 2305 breast tumours. Breast Cancer Res. 2019, 21, 34. [Google Scholar] [CrossRef] [Green Version]
- Koboldt, D.C.; Fulton, R.S.; McLellan, M.D.; Schmidt, H.; Kalicki-Veizer, J.; McMichael, J.F.; Fulton, L.L.; Dooling, D.J.; Ding, L.; Mardis, E.R.; et al. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar]
- Geradts, J.; Wilson, P.A. High frequency of aberrant p16(INK4A) expression in human breast cancer. Am. J. Pathol. 1996, 149, 15–20. [Google Scholar] [PubMed]
- Turner, N.C.; André, F.; Loi, S.; Verma, S.; Iwata, H.; Harbeck, N.; Loibl, S.; Bartlett, C.H.; Zhang, K.; et al.; Jungsil PALOMA3 Study Group Palbociclib in Hormone-Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2015, 373, 209–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, C. Pfizer’s CDK4/6 inhibitor approved for advanced breast cancer. Nat. Biotechnol. 2015, 33, 323–324. [Google Scholar] [CrossRef]
- Gao, J.J.; Cheng, J.; Bloomquist, E.; Sanchez, J.; Wedam, S.B.; Singh, H.; Amiri-Kordestani, L.; Ibrahim, A.; Sridhara, R.; Goldberg, K.B.; et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: A US Food and Drug Administration pooled analysis. Lancet Oncol. 2020, 21, 250–260. [Google Scholar] [CrossRef]
- Finn, R.S.; Crown, J.P.; Lang, I.; Boér, K.; Bondarenko, I.M.; O Kulyk, S.; Ettl, J.; Patel, R.; Pinter, T.; Schmidt, M.; et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015, 16, 25–35. [Google Scholar] [CrossRef]
- Tripathy, D.; Im, S.-A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.; Burris, H.; Yap, Y.; Sonke, G.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.; Winer, E.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541–1547. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef]
- Baselga, J.; Cortés, J.; Kim, S.-B.; Im, S.-A.; Hegg, R.; Im, Y.-H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Baselga, J.; Semiglazov, V.; Van Dam, P.; Manikhas, A.; Bellet, M.; Mayordomo, J.; Campone, M.; Kubista, E.; Greil, R.; Bianchi, G.; et al. Phase II Randomized Study of Neoadjuvant Everolimus Plus Letrozole Compared With Placebo Plus Letrozole in Patients With Estrogen Receptor–Positive Breast Cancer. J. Clin. Oncol. 2009, 27, 2630–2637. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Chen, D.D.; Piccart-Gebhart, M.; Rugo, H.S.; Burris, H.H.; Pritchard, K.I.; Campone, M.; Noguchi, S.S.; Perez, A.T.; Deleu, I.I.; et al. Correlative Analysis of Genetic Alterations and Everolimus Benefit in Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer: Results From BOLERO-2. J. Clin. Oncol. 2016, 34, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Chandarlapaty, S.; Chen, D.; He, W.; Sung, P.; Samoila, A.; You, D.; Bhatt, T.; Patel, P.; Voi, M.; Gnant, M.; et al. Prevalence of ESR1 Mutations in Cell-Free DNA and Outcomes in Metastatic Breast Cancer. JAMA Oncol. 2016, 2, 1310–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, I.A.; Abramson, V.G.; Formisano, L.; Balko, J.M.; Estrada, M.V.; Sanders, M.E.; Juric, D.; Solit, D.; Berger, M.F.; Won, H.H.; et al. A Phase Ib Study of Alpelisib (BYL719), a PI3Kα-Specific Inhibitor, with Letrozole in ER+/HER2− Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Mayer, I.A.; Prat, A.; Egle, D.; Blau, S.; Fidalgo, J.A.P.; Gnant, M.; Fasching, P.A.; Colleoni, M.; Wolff, A.C.; Winer, E.P.; et al. A Phase II Randomized Study of Neoadjuvant Letrozole Plus Alpelisib for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer (NEO-ORB). Clin. Cancer Res. 2019, 25, 2975–2987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juric, D.; Baselga, J. Tumor Genetic Testing for Patient Selection in Phase I Clinical Trials: The Case of PI3K Inhibitors. J. Clin. Oncol. 2012, 30, 765–766. [Google Scholar] [CrossRef]
- Arnedos, M.; Vicier, C.; Loi, S.; Lefebvre, C.; Michiels, S.; Bonnefoi, H.; Andre, F. Precision medicine for metastatic breast cancer—limitations and solutions. Nat. Rev. Clin. Oncol. 2015, 12, 693–704. [Google Scholar] [CrossRef]
- Krop, E.I.; Mayer, I.I.; Ganju, V.V.; Dickler, M.M.; Johnston, S.; Morales, S.S.; Yardley, D.D.; Melichar, B.B.; Forero-Torres, A.A.; Lee, S.C.S.; et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016, 17, 811–821. [Google Scholar] [CrossRef] [Green Version]
- Dent, S.; Cortés, J.; Im, Y.-H.; Diéras, V.; Harbeck, N.; Krop, I.; Wilson, T.; Cui, N.; Schimmoller, F.; Hsu, J.; et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: The SANDPIPER trial. Ann. Oncol. 2021, 32, 197–207. [Google Scholar] [CrossRef]
- Baselga, J.; Im, S.A.S.; Iwata, H.; Cortés, J.; De Laurentiis, M.; Jiang, Z.; Arteaga, C.C.; Jonat, W.; Clemons, M.J.; Ito, Y.Y.; et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 904–916. [Google Scholar] [CrossRef]
- Blake, J.F.; Xu, R.; Bencsik, J.R.; Xiao, D.; Kallan, N.C.; Schlachter, S.; Mitchell, I.S.; Spencer, K.L.; Banka, A.L.; Wallace, E.M.; et al. Discovery and Preclinical Pharmacology of a Selective ATP-Competitive Akt Inhibitor (GDC-0068) for the Treatment of Human Tumors. J. Med. Chem. 2012, 55, 8110–8127. [Google Scholar] [CrossRef]
- Karlsson, E.; Pongsawasdi, P. Purification of two phospholipase A isoenzymes with anticoagulant activity from the venom of the cobra Naja naja siamensis. Toxicon 1980, 18, 409–419. [Google Scholar] [CrossRef]
- Brown, J.S.; Banerji, U. Maximising the potential of AKT inhibitors as anti-cancer treatments. Pharmacol. Ther. 2017, 172, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.-H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef] [Green Version]
- Malachi, G.; Suman, V.; Goetz, M.P.; Northfelt, D.; Burkard, M.E.; Ademuyiwa, F.; Naughton, M.; Margenthaler, J.; Aft, R.; Gray, R.; et al. A Phase II Trial of Neoadjuvant MK-2206, an AKT Inhibitor, with Anastrozole in Clinical Stage II or III PIK3CA-Mutant ER-Positive and HER2-Negative Breast Cancer. Clin. Cancer Res. 2017, 23, 6823–6832. [Google Scholar] [CrossRef] [Green Version]



| Class of Drug | Mechanism of Action | Drugs Approved for Clinical Use | Drugs in Clinical Development |
|---|---|---|---|
| Selective estrogen receptor modulators (SERMs) | Suppression of E2-regulated gene expression by enhancing corepressor recruitment to ERα [56] | Tamoxifen, Toremifene, Raloxifene [62] | Bazedoxifene [62,63] |
| Selective estrogen receptor downregulators (SERDs) | SERDs disrupt ER dimerization and DNA binding and aid premature proteosomal degradation of the receptor [64] | Fulvestrant [57,65,66] | Elacestrant (RAD1901), AZD-9496, GDC-0927, LSZ102, SAR439859, G1T48 [66,67,68,69,70] |
| Aromatase inhibitors (AIs) | AIs prevent aromatase-mediated synthesis of estrogens from androgens. Thereby, decreasing circulating estrogen levels [71] | Exemestane (steroidal), Letrozole, Anastrozole (nonsteroidal) [72] |
| Current Strategies | FDA Approved Drugs/Drug Combinations and Associated Clinical Trials | Drugs/Drug Combinations in Clinical Trials |
|---|---|---|
| Target mutant ERα through new class of SERDs | Fulvestrant [75] | AZD9496, GDC0927, RAD1901, GDC0810 [76,77] |
| Inhibition of CCND1-CDK4/6-RB pathway | Ribociclib (LEE011) in combination with endocrine therapy (Tamoxifen and Goserelin or a nonsteroidal aromatase inhibitor and Goserelin) (MONALEESA-7 trial) [85] | |
| Inhibition of the PI3K-AKT-mTOR pathway |
| Ipatasertib in combination with endocrine therapy and a CDK4/6 inhibitor (TAKTIC trial) [89] |
| Concurrent inhibition of ERα, CCND1-CDK4/6-RB pathway and the PI3K-AKT-mTOR pathways | Triplet therapy combining Palbociclib, Taselisib and Fulvestrant and doublet therapy combining Palbociclib and Taselisib [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatpe, A.S.; Adebayo, A.K.; Herodotou, C.A.; Kumar, B.; Nakshatri, H. Nexus between PI3K/AKT and Estrogen Receptor Signaling in Breast Cancer. Cancers 2021, 13, 369. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13030369
Khatpe AS, Adebayo AK, Herodotou CA, Kumar B, Nakshatri H. Nexus between PI3K/AKT and Estrogen Receptor Signaling in Breast Cancer. Cancers. 2021; 13(3):369. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13030369
Chicago/Turabian StyleKhatpe, Aditi S., Adedeji K. Adebayo, Christopher A. Herodotou, Brijesh Kumar, and Harikrishna Nakshatri. 2021. "Nexus between PI3K/AKT and Estrogen Receptor Signaling in Breast Cancer" Cancers 13, no. 3: 369. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13030369