Early PSA Change after [177Lu]PSMA-617 Radioligand Therapy as a Predicator of Biochemical Response and Overall Survival
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
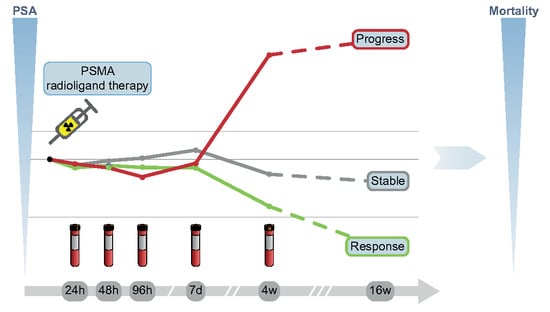
2.2. Treatment Regime and PSA Measurement
2.3. Efficacy and Response/Endpoints
2.4. Statistical Analysis
3. Results
3.1. Early PSA Change with Regard to Biochemical Response
3.1.1. Early PSA Change and Biochemical Response after 16 Weeks
3.1.2. Biochemical Response Categorization by Early PSA Change
3.1.3. Exploratory Retrospective ROC Analysis
3.2. Early PSA Change with Regard to Change in Tumour Volume
3.3. Early PSA Change with Regard to Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, A.; Heston, W.D.W. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J. Cell Biochem. 2004, 91, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Violet, J.; Sandhu, S.; Iravani, A.; Ferdinandus, J.; Thang, S.-P.; Kong, G.; Kumar, A.R.; Akhurst, T.; Pattison, D.A.; Beaulieu, A.; et al. Long-term follow-up and outcomes of retreatment in an expanded 50-patient single-center phase II prospective trial of 177Lu-PSMA-617 theranostics in metastatic castration-resistant prostate cancer. J. Nucl. Med. 2020, 61, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schäfers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. 177Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the prostate cancer clinical trials working group 3. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef] [Green Version]
- Gafita, A.; Fendler, W.P.; Hui, W.; Sandhu, S.; Weber, M.; Esfandiari, R.; Calais, J.; Rauscher, I.; Rathke, H.; Tauber, R.; et al. Efficacy and safety of 177Lu-labeled prostate-specific membrane antigen radionuclide treatment in patients with diffuse bone marrow involvement: A multicenter retrospective study. Eur. Urol. 2020, 78, 148–154. [Google Scholar] [CrossRef]
- Fanti, S.; Goffin, K.; Hadaschik, B.A.; Herrmann, K.; Maurer, T.; MacLennan, S.; Oprea-Lager, D.E.; Oyen, W.J.; Rouvière, O.; Mottet, N.; et al. Consensus statements on PSMA PET/CT response assessment criteria in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 469–476. [Google Scholar] [CrossRef]
- Gafita, A.; Heck, M.M.; Rauscher, I.; Tauber, R.; Cala, L.; Franz, C.; D’Alessandria, C.; Retz, M.; Weber, W.A.; Eiber, M. Early prostate-specific antigen changes and clinical outcome after 177Lu-PSMA radionuclide treatment in patients with metastatic castration-resistant prostate cancer. J. Nucl. Med. 2020, 61, 1476–1483. [Google Scholar] [CrossRef]
- Angelergues, A.; Maillet, D.; Fléchon, A.; Ozgüroglu, M.; Mercier, F.; Guillot, A.; Le Moulec, S.; Gravis, G.; Beuzeboc, P.; Massard, C.; et al. Prostate-specific antigen flare induced by cabazitaxel-based chemotherapy in patients with metastatic castration-resistant prostate cancer. Eur. J. Cancer 2014, 50, 1602–1609. [Google Scholar] [CrossRef]
- Berthold, D.R.; Pond, G.R.; Soban, F.; de Wit, R.; Eisenberger, M.; Tannock, I.F. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: Updated survival in the TAX 327 study. J. Clin. Oncol. 2008, 26, 242–245. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Wegen, S.; Yordanova, A.; Fimmers, R.; Kürpig, S.; Eppard, E.; Wei, X.; Schlenkhoff, C.; Hauser, S.; Essler, M. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using 177Lu-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1448–1454. [Google Scholar] [CrossRef]
- Rescigno, P.; Dolling, D.; Conteduca, V.; Rediti, M.; Bianchini, D.; Lolli, C.; Ong, M.; Li, H.; Omlin, A.G.; Schmid, S.; et al. Early post-treatment prostate-specific antigen at 4 weeks and abiraterone and enzalutamide treatment for advanced prostate cancer: An international collaborative analysis. Eur. Urol. Oncol. 2020, 3, 176–182. [Google Scholar] [CrossRef]
- Atakan, D.; Ozkan, A.; Guve, M.A.; Sinan, K. Early PSA response to antiandrogen therapy in metastatic castration-resistant prostate carcinoma patients: A predictive marker for progression-free survival? J. Balk. Union Oncol. 2020, 25, 1625–1630. [Google Scholar]
- Stamey, T.A.; Yang, N.; Hay, A.R.; McNeal, J.E.; Freiha, F.S.; Redwine, E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N. Engl. J. Med. 1987, 317, 909–916. [Google Scholar] [CrossRef]
- Kim, J.S.; Ryu, J.-G.; Kim, J.W.; Hwang, E.C.; Jung, S.I.; Kang, T.W.; Kwon, D.; Park, K. Prostate-specific antigen fluctuation: What does it mean in diagnosis of prostate cancer? Int. Braz. J. Urol. 2015, 41, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Fendler, W.P.; Kratochwil, C.; Ahmadzadehfar, H.; Rahbar, K.; Baum, R.P.; Schmidt, M.; Pfestroff, A.; Lützen, U.; Prasad, V.; Heinzel, A.; et al. 177Lu-PSMA-617 therapy, dosimetry and follow-up in patients with metastatic castration-resistant prostate cancer. Nuklearmedizin 2016, 55, 123–128. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Delgado Bolton, R.C.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Rahbar, K.; Kürpig, S.; Bögemann, M.; Claesener, M.; Eppard, E.; Gärtner, F.; Rogenhofer, S.; Schäfers, M.; Essler, M. Early side effects and first results of radioligand therapy with 177Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: A two-centre study. EJNMMI Res. 2015, 5, 114. [Google Scholar] [CrossRef] [Green Version]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Halabi, S.; Armstrong, A.J.; Sartor, O.; de Bono, J.; Kaplan, E.; Lin, C.-Y.; Solomon, N.C.; Small, E.J. Prostate-specific antigen changes as surrogate for overall survival in men with metastatic castration-resistant prostate cancer treated with second-line chemotherapy. J. Clin. Oncol. 2013, 31, 3944–3950. [Google Scholar] [CrossRef] [Green Version]
- Heck, M.M.; Tauber, R.; Schwaiger, S.; Retz, M.; D’Alessandria, C.; Maurer, T.; Gafita, A.; Wester, H.-J.; Gschwend, J.E.; Weber, W.A.; et al. Treatment outcome, toxicity, and predictive factors for radioligand therapy with 177Lu-PSMA-I&T in metastatic castration-resistant prostate cancer. Eur. Urol. 2019, 75, 920–926. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Kanoun, S.; Tal, I.; Jean Marc, V.; Cochet, A. Beth Israel plugin for FIJI: Logiciel gratuit et open source pour la recherche scientifique. Méd. Nucl. 2016, 40, 194. [Google Scholar] [CrossRef]
- Seifert, R.; Herrmann, K.; Kleesiek, J.; Schäfers, M.; Shah, V.; Xu, Z.; Chabin, G.; Grbic, S.; Spottiswoode, B.; Rahbar, K. Semiautomatically quantified tumor volume using 68Ga-PSMA-11 PET as a biomarker for survival in patients with advanced prostate cancer. J. Nucl. Med. 2020, 61, 1786–1792. [Google Scholar] [CrossRef]
- Ueda, Y.; Matsubara, N.; Tabata, K.-I.; Satoh, T.; Kamiya, N.; Suzuki, H.; Kawahara, T.; Uemura, H. Prostate-specific antigen flare phenomenon induced by abiraterone acetate in chemotherapy-naive patients with metastatic castration-resistant prostate cancer. Clin. Genitourin. Cancer 2017, 15, 320–325. [Google Scholar] [CrossRef]
- Ferrell, B.; Connor, S.R.; Cordes, A.; Dahlin, C.M.; Fine, P.G.; Hutton, N.; Leenay, M.; Lentz, J.; Person, J.L.; Meier, D.E.; et al. The National Agenda for Quality Palliative Care: The National Consensus Project and the National Quality Forum. J. Pain Symptom Manag. 2007, 33, 737–744. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Ankerst, D.P.; Jiang, C.S.; Tangen, C.M.; Hussain, M.H.A.; Lara, P.N.; Jones, J.A.; Taplin, M.E.; Burch, P.A.; Kohli, M.; et al. Evaluation of prostate-specific antigen declines for surrogacy in patients treated on SWOG 99-16. J. Natl. Cancer Inst. 2006, 98, 516–521. [Google Scholar] [CrossRef] [Green Version]
- Rahbar, K.; Boegemann, M.; Yordanova, A.; Eveslage, M.; Schäfers, M.; Essler, M.; Ahmadzadehfar, H. PSMA targeted radioligandtherapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 12–19. [Google Scholar] [CrossRef] [PubMed]





| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| p | HR (95% CI) | p | HR (95% CI) | |
| UICC Stage at diagnosis | 0.423 | |||
| Postsurgical Gleason Score | 0.854 | |||
| Previous Treatments | ||||
| Radical Prostatectomy | 0.586 | |||
| Radiotherapy | 0.55 | |||
| Androgen Deprivation | 0.41 | |||
| Abiraterone | 0.571 | |||
| Enzalutamide | 0.63 | |||
| [223Ra]Radiumdichloride | 0.111 | |||
| Docetaxel | 0.564 | |||
| Cabazitaxel | 0.04 | 7.22 (1.7–30.54) | 0.893 | |
| number of previous systemic therapy lines * | 0.904 | |||
| Age | 0.723 | |||
| Lymph Node Metastases | 0.471 | |||
| Performance Status (Karnofsky index) | 0.012 | 0.91 (0.85–0.98) | 0.156 | |
| HB (g/dL) | 0.002 | 0.74 (0.61–0.90) | 0.249 | |
| LDH (U/L) | 0.002 | 1.004 (1.001–1.007) | 0.428 | |
| PSA (ng/mL) | 0.034 | 1.76 (1.34–2.47) | 0.012 | 1.78 (1.03–3.09) |
| Tumor volume on PET | 0.01 | 1.004 (1.002–1.006) | 0.014 | 1.003 (1.001–1.006) |
| Δ%PSA 4 Weeks after First Administration | Biochemical Response at Restaging According to PCWG3 Criteria | |||
|---|---|---|---|---|
| Response a | Stable b | Progression c | Total | |
| Δ%PSA ≤ −30% | 10 (91%) | 1 (9%) | - | 11 |
| −30% < Δ%PSA < 25% | 6 (67%) | 2 (22%) | 1 (11%) | 9 |
| Δ%PSA ≥ 25% | - | - | 3 (100%) | 3 |
| Total | 16 | 3 | 4 | n = 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kind, F.; Fassbender, T.F.; Andrieux, G.; Boerries, M.; Meyer, P.T.; Ruf, J. Early PSA Change after [177Lu]PSMA-617 Radioligand Therapy as a Predicator of Biochemical Response and Overall Survival. Cancers 2022, 14, 149. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14010149
Kind F, Fassbender TF, Andrieux G, Boerries M, Meyer PT, Ruf J. Early PSA Change after [177Lu]PSMA-617 Radioligand Therapy as a Predicator of Biochemical Response and Overall Survival. Cancers. 2022; 14(1):149. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14010149
Chicago/Turabian StyleKind, Felix, Thomas F. Fassbender, Geoffroy Andrieux, Melanie Boerries, Philipp T. Meyer, and Juri Ruf. 2022. "Early PSA Change after [177Lu]PSMA-617 Radioligand Therapy as a Predicator of Biochemical Response and Overall Survival" Cancers 14, no. 1: 149. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14010149