Autologous Stem-Cell Transplantation for High-Risk Neuroblastoma: Historical and Critical Review
Abstract
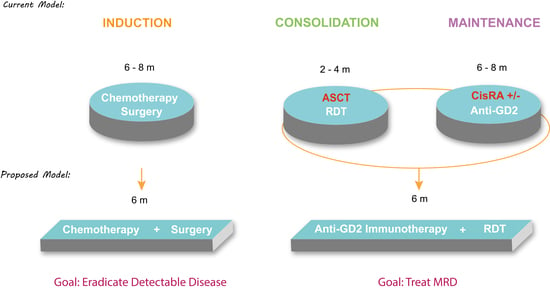
:Simple Summary
Abstract
1. Hematopoietic Stem-Cell Transplant: A Revolutionary Development from the 20th Century for Hematological Diseases. Historical Review
2. The Basic Principle of Transplantation Expanded to Solid Tumors: More Is Not always Better
3. Why Only Neuroblastoma? The Original Mistake
4. Event-Free Survival and Overall Survival
5. Anti-GD2 Immunotherapy and ASCT
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Jacobson, L.O.; Marks, E.K.; Robson, M.J.; Gaston, E.O.; Zirkle, R.E. Effect of spleen protection on mortality following X-irradiation. J. Lab. Clin. Med. 1949, 34, 1538–1543. [Google Scholar]
- Lorenz, E.; Uphoff, D.; Reid, T.R.; Shelton, E. Modification of irradiation injury in mice and guinea pigs by bone marrow injections. J. Natl. Cancer Inst. 1951, 12, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.D.; Lochte, H.L., Jr.; Cannon, J.H.; Sahler, O.D.; Ferrebee, J.W. Supralethal whole body irradiation and isologous marrow transplantation in man. J. Clin. Investig. 1959, 38, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Mathé, G.; Amiel, J.L.; Schwarzenberg, L.; Catton, A.; Schneider, M. Adoptive immunotherapy of acute leukemia: Experimental and clinical results. Cancer Res. 1965, 25, 1525–1531. [Google Scholar]
- McGovern, J.J., Jr.; Russel, P.S.; Atkins, L.; Webster, E.W. Treatment of terminal leukemic relapse by total-body irradiation and intravenous infusion of stored autologous bone marrow obtained during remission. N. Engl. J. Med. 1959, 260, 675–683. [Google Scholar] [CrossRef]
- Santos, G.W.; Sensenbrenner, L.L.; Burke, P.J.; Colvin, M.; Owens Jr, A.H.; Bias, W.B.; Slavin, R.E. Marrow transplantation in man following cyclophosphamide. Transplant. Proc. 1971, 3, 400–404. [Google Scholar]
- Santos, G.W. Busulfan (Bu) and cyclophosphamide (Cy) or marrow transplantation. Bone Marrow Transplant. 1989, 4, 236–239. [Google Scholar]
- Frei, E., III; Teicher, B.A.; Holden, S.A.; Cathcart, K.N.; Wang, Y.Y. Preclinical studies and clinical correlation of the effect of alkylating dose. Cancer Res. 1988, 48, 6417–6423. [Google Scholar]
- Frei, E.J.; Canellos, G.P. Dose: A critical factor in cancer chemotherapy. Am. J. Med. 1980, 69, 585–594. [Google Scholar] [CrossRef]
- Frei, E., III; Antman, K.; Teicher, B.; Eder, P.; Schnipper, L. Bone marrow autotransplantation for solid tumors prospects. J. Clin. Oncol. 1989, 7, 515–526. [Google Scholar] [CrossRef]
- MacNeil, M.; Eisenhauer, E.A. Adults: High-dose chemotherapy: Is it standard management for any common solid tumor? Ann. Oncol. 1999, 10, 1145–1161. [Google Scholar] [CrossRef] [PubMed]
- Livingston, R.B. Dose intensity and high dose therapy. Cancer 1994, 74, 1177–1182. [Google Scholar] [CrossRef]
- Marina, N.M.; Pappo, A.S.; Parham, D.M.; Cain, A.M.; Rao, B.N.; Poquette, C.A.; Pratt, C.B.; Greenwald, C.; Meyer, W.H. Chemotherapy dose-intensification for pediatric patients with Ewing’s family of tumors and desmoplastic small round-cell tumors: A feasibility study at St. Jude Children’s Research Hospital. J. Clin. Oncol. 1999, 17, 180–190. [Google Scholar] [CrossRef]
- Whelan, J.; Khan, A.; Sharma, A.; Rothermundt, C.; Dileo, P.; Michelagnoli, M.; Seddon, B.; Strausss, S. Interval compressed vincristine, doxorubicin, cyclophosphamide alternating with ifosfamide, etoposide in patients with advanced Ewing’s and other Small Round Cell Sarcomas. Clin. Sarcoma Res. 2012, 21, 12. [Google Scholar] [CrossRef] [Green Version]
- Cheung, N.-K.V.; Heller, G. Chemotherapy Dose Intensity Correlates Strongly With Response, Median Survival, and Median Progression-Free Survival in Metastatic Neuroblastoma. J. Clin. Oncol. 1991, 9, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Savarese, D.M.F.; Hsieh, C.-C.; Stewart, F.M. Clinical Impact of Chemotherapy Dose Escalation in Patients With Hematologic Malignancies and Solid Tumors. J. Clin. Oncol. 1997, 15, 2981–2995. [Google Scholar] [CrossRef]
- Rettig, R.A.; Jacobson, P.D.; Farquhar, C.M.; Aubry, W.M. False Hope: Bone Marrow Transplantation for Breast Cancer; Oxford University Press: London, UK, 2007; ISBN 978-0195187762. [Google Scholar]
- Haveman, L.M.; van Ewijk, R.; van Dalen, E.C.; Breunis, W.B.; Kremer, L.C.; van den Berg, H.; Dirksen, U.; Merks, J.H. High-dose chemotherapy followed by autologous haematopoietic cell transplantation for children, adolescents, and young adults with primary metastatic Ewing sarcoma. Cochrane Database Syst. Rev. 2021, 9, CD011405. [Google Scholar]
- Delafoy, M.; Verschuur, A.; Scheleirmacher, G.; Tabone, M.D.; Sudour-Bonnange, H.; Thébaud, E.; Freycon, C.; Notz-Carrère, A.; Boulanger, C.; Pellier, I.; et al. High-dose chemotherapy followed by autologous stem cell rescue in Wilms tumors: French report on toxicity and efficacy. Pediatric Blood Cancer 2022, 69, e29431. [Google Scholar] [CrossRef]
- Peinemann, F.; Enk, H.; Smith, L.A. Autologous hematopoietic stem cell transplantation following high-dose chemotherapy for nonrhabdomyosarcoma soft tissue sarcomas. Cochrane Database Syst. Rev. 2017, 4, CD008216. [Google Scholar] [CrossRef]
- Peinemann, F.; Kröger, N.; Bartel, C.; Grouven, U.; Pittler, M.; Erttmann, R.; Kulig, M. High-dose chemotherapy followed by autologous stem cell transplantation for metastatic rhabdomyosarcoma--a systematic review. PLoS ONE 2011, 6, e17127. [Google Scholar] [CrossRef] [Green Version]
- Sauerbrey, A.; Bielack, S.; Kempf-Bielack, B.; Zoubek, A.; Paulussen, M.; Zintl, F. High-dose chemotherapy (HDC) and autologous hematopoietic stem cell transplantation (ASCT) as salvage therapy for relapsed osteosarcoma. Bone Marrow Transplant. 2001, 27, 933–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häberle, B.; Maxwell, R.; von Schweinitz, D.; Schmid, I. High Dose Chemotherapy with Autologous Stem Cell Transplantation in Hepatoblastoma does not Improve Outcome. Results of the GPOH Study HB99. Klin. Pädiatrie 2019, 231, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Furtwängler, R.; Kager, L.; Melchior, P.; Rübe, C.; Ebinger, M.; Nourkami-Tutdibi, N.; Niggli, F.; Warmann, S.; Hubertus, J.; Amman, G.; et al. High-dose treatment for malignant rhabdoid tumor of the kidney: No evidence for improved survival-The Gesellschaft für Pädiatrische Onkologie und Hämatologie (GPOH) experience. Pediatric Blood Cancer 2018, 65. [Google Scholar] [CrossRef] [PubMed]
- Dini, G.; Philip, T.; Hartmann, O.; Pinkerton, R.; Chauvin, F.; Garaventa, A.; Lanino, E.; Dallorso, S. Bone marrow transplantation for neuroblastoma: A review of 509 cases. Bone Marrow Transplant. 1989, 4 (Suppl. 4), 42–46. [Google Scholar] [PubMed]
- Dini, G.; Lanino, E.; Garaventa, A.; Rogers, D.; Dallorso, S.; Viscoli, C.; Castagnola, E.; Manno, G.; Brisigotti, M.; Rosanda, C.; et al. Myeloablative therapy and unpurged autologous bone marrow transplantation for poor-prognosis neuroblastoma: Report of 34 cases. J. Clin. Oncol. 1991, 9, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Philip, T.; Zucker, J.M.; Bernard, J.L.; Lutz, P.; Bordigoni, P.; Plouvier, E.; Robert, A.; Roché, H.; Souillet, G.; Bouffet, E.; et al. Improved survival at 2 and 5 years in the LMCE1 unselected group of 72 children with stage IV neuroblastoma older than 1 year of age at diagnosis: Is cure possible in a small subgroup? J. Clin. Oncol. 1991, 9, 1037–1044. [Google Scholar] [CrossRef]
- Kushner, B.H.; O’Reilly, R.J.; Mandell, L.R.; Gulati, S.C.; LaQuaglia, M.; Cheung, N.K. Myeloablative combination chemotherapy without total body irradiation for neuroblastoma. J. Clin. Oncol. 1991, 9, 274–279. [Google Scholar] [CrossRef]
- Seeger, R.C.; Reynolds, C.P. Treatment of high-risk solid tumors of childhood with intensive therapy and autologous bone marrow transplantation. Pediatric Clin. N. Am. 1991, 38, 393–424. [Google Scholar] [CrossRef]
- Matthay, K.K.; Atkinson, J.B.; Stram, D.O.; Selch, M.; Reynolds, C.P.; Seeger, R.C. Patterns of relapse after autologous purged bone marrow transplantation for neuroblastoma: A Childrens Cancer Group pilot study. J. Clin. Oncol. 1993, 11, 2226–2233. [Google Scholar] [CrossRef]
- Ohnuma, N.; Takahashi, H.; Kaneko, M.; Uchino, J.; Takeda, T.; Iwafuchi, M.; Ohhira, M.; Nishihira, H.; Mugishima, H.; Yokoyama, J.; et al. Treatment combined with bone marrow transplantation for advanced neuroblastoma: An analysis of patients who were pretreated intensively with the protocol of the Study Group of Japan. Med. Pediatric Oncol. 1995, 24, 181–187. [Google Scholar] [CrossRef]
- Garaventa, A.; Rondelli, R.; Lanino, E.; Dallorso, S.; Dini, G.; Bonetti, F.; Arrighini, A.; Santoro, N.; Rossetti, F.; Miniero, F.; et al. Myeloablative therapy and bone marrow rescue in advanced neuroblastoma: Report from the Italian Bone Marrow Transplant Registry. Bone Marrow Transplant. 1996, 18, 125–130. [Google Scholar] [PubMed]
- Stram, D.O.; Matthay, K.K.; O’Leary, M.; Reynolds, C.P.; Haase, G.M.; Atkinson, J.B.; Brodeur, G.M.; Seeger, R.C. Consolidation chemoradiotherapy and autologous bone marrow transplantation versus continued chemotherapy for metastatic neuroblastoma: A report of two concurrent Children’s Cancer Group studies. J. Clin. Oncol. 1996, 14, 2417–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushner, B.H.; Gulati, S.C.; O’Reilly, R.J.; Heller, G.; Cheung, N.-K.V. Autografting with bone marrow exposed to multiple courses of very high dose cyclophosphamide in vivo and to 4-hydroperoxy-cyclophosphamide in vitro. Med. Pediatric Oncol. 1990, 18, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Kushner, B.H.; Hadju, S.I.; Gulati, S.C.; Erlandson, R.A.; Exelby, P.R.; Lieberman, P.H. Extracranial primitive neuroectodermal tumors: The Memorial Sloan-Kettering Cancer Center experience. Cancer 1991, 67, 1825–1829. [Google Scholar] [CrossRef]
- Kushner, B.H.; Gulati, S.C.; Kwon, J.H.; O’Reilly, R.J.; Exelby, P.R.; Cheung, N.-K.V. High-dose melphalan with 6-hydroxydopamine-purged autologous bone marrow transplantation for poor-risk neuroblas-toma. Cancer 1991, 68, 242–247. [Google Scholar] [CrossRef]
- Kushner, B.H.; Cheung, N.-K.V.; Kramer, K.; Dunkel, I.J.; Calleja, E.; Boulad, F. Topotecan combined with myeloablative doses of thiotepa and carboplatin for neuroblastoma, brain tumors, and other poor-risk solid tumors in children and young adults. Bone Marrow Transplant. 2001, 28, 551–556. [Google Scholar] [CrossRef] [Green Version]
- Kushner, B.H.; Kramer, K.; Modak, S.; Kernan, N.A.; Reich, L.M.; Danis, K.; Cheung, N.-K.V. Topotecan, thiotepa, and carboplatin for neuroblastoma: Failure to prevent relapse in the central nervous system. Bone Marrow Transplant. 2006, 37, 271–276. [Google Scholar] [CrossRef]
- Fish, J.D.; Grupp, S.A. Stem cell transplantation for neuroblastoma. Bone Marrow Transplant. 2008, 41, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Matthay, K.K.; Villablanca, J.G.; Seeger, R.C.; Stram, D.O.; Harris, R.E.; Ramsay, N.K.; Swift, P.; Shimada, H.; Black, C.T.; Brodeur, G.M.; et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N. Engl. J. Med. 1999, 341, 1165–1173. [Google Scholar] [CrossRef]
- Pritchard, J.; Cotterill, S.J.; Germond, S.M.; Imeson, J.; de Kraker, J.; Jones, D.R. High dose melphalan in the treatment of advanced neuroblastoma: Results of a randomised trial (ENSG-1) by the European Neuroblastoma Study Group. Pediatric Blood Cancer 2005, 44, 348–357. [Google Scholar] [CrossRef]
- Berthold, F.; Boos, J.; Burdach, S.; Erttmann, R.; Henze, G.; Hermann, J.; Klingebiel, T.; Kremens, B.; Schilling, F.H.; Schrappe, M.; et al. Myeloablative megatherapy with autologous stem-cel rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: A randomised controlled trial. Lancet Oncol. 2005, 6, 649–658. [Google Scholar] [CrossRef]
- Matthay, K.K.; Reynolds, C.P.; Seeger, R.C.; Shimada, H.; Adkins, E.S.; Haas-Kogan, D.; Gerbing, R.B.; London, W.B.; Villablanca, J.G. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A children’s oncology group study. J. Clin. Oncol. 2009, 27, 1007–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthay, K.K.; Reynolds, C.P.; Seeger, R.C.; Shimada, H.; Adkins, E.S.; Haas-Kogan, D.; Gerbing, R.B.; London, W.B.; Villablanca, J.G. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A children’s oncology group study. J. Clin. Oncol. 2009, 27, 1007–1013, Erratum in J. Clin. Oncol. 2014, 32, 1862–1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.R.; Kreissman, S.G.; London, W.B.; Naranjo, A.; Cohn, S.L.; Hogarty, M.D.; Tenney, S.C.; Haas-Kogan, D.; Shaw, P.J.; Kraveka, J.M.; et al. Effect of Tandem Autologous Stem Cell Transplant vs Single Transplant on Event-Free Survival in Patients With High-Risk Neuroblastoma: A Randomized Clinical Trial. JAMA 2019, 322, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, B.; Kremer, L.C.; Caron, H.N.; van Dalen, E.C. High-dose chemotherapy and autologous haematopoietic stem cell rescue for children with high-risk neuroblastoma. Cochrane Database Syst Rev. 2013, 8, CD006301. [Google Scholar] [CrossRef] [Green Version]
- Yalçin, B.; Kremer, L.C.; van Dalen, E.C. High-dose chemotherapy and autologous haematopoietic stem cell rescue for children with high-risk neuroblastoma. Cochrane Database Syst Rev. 2015, 5, CD006301. [Google Scholar] [CrossRef]
- Kreissman, S.G.; Seeger, R.C.; Matthay, K.K.; London, W.B.; Sposto, R.; Grupp, S.A.; Haas-Kogan, D.A.; Laquaglia, M.P.; Yu, A.L.; Diller, L.; et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 999–1008. [Google Scholar] [CrossRef] [Green Version]
- Ellis, L.M.; Bernstein, D.S.; Voest, E.E.; Berlin, J.D.; Sargent, D.; Cortazar, P.; Garrett-Mayer, E.; Herbst, R.S.; Lilenbaum, R.C.; Sima, C.; et al. American Society of Clinical Oncology perspective: Raising the bar for clinical trials by defining clinically meaningful outcomes. J. Clin. Oncol. 2014, 32, 1277–1280. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.; Schneiderman, J.; Helenowski, I.B.; Morgan, E.; Dilley, K.; Danner-Koptik, K.; Hatahet, M.; Shimada, H.; Cohn, S.L.; Kletzel, M.; et al. Secondary malignant neoplasms after high-dose chemotherapy and autologous stem cell rescue for high-risk neuroblasto-ma. Pediatric Blood Cancer 2014, 61, 1350–1356. [Google Scholar] [CrossRef]
- Coorens, T.H.H.; Collord, G.; Lu, W.; Mitchell, E.; Ijaz, J.; Roberts, T.; Oliver, T.R.W.; Burke, G.A.A.; Gattens, M.; Dickens, E.; et al. Clonal hematopoiesis and therapy-related myeloid neoplasms following neuroblastoma treatment. Blood 2021, 137, 2992–2997. [Google Scholar] [CrossRef]
- Rubino, C.; Adjadj, E.; Guerin, S.; Guibout, C.; Shamsaldin, A.; Dondon, M.G.; Valteau-Couanet, D.; Hartmann, O.; Hawkins, M.; de Vathaire, F. Long-term risk of second malignant neoplasms after neuroblastoma in childhood: Role of treatment. Int. J. Cancer 2003, 107, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Laverdiere, C.; Liu, Q.; Yasui, Y.; Nathan, P.C.; Gurney, J.G.; Stovall, M.; Diller, L.R.; Cheung, N.-K.; Wolden, S.; Robison, L.L.; et al. Long-term outcomes in survivors of neuroblastoma: A report from the Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2009, 101, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Kushner, B.H.; Cheung, N.K.; Kramer, K.; Heller, G.; Jhanwar, S.C. Neuroblastoma and treatment-related myelodysplasia/leukemia: The Memorial Sloan-Kettering experience and a literature review. J. Clin. Oncol. 1998, 16, 3880–3889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, N.; Scobie, N.; Palmer, A.; Ludwinski, D. To transplant, or not to transplant? That is the question. A patient advocate evaluation of autologous stem cell transplant in neuroblastoma. Pediatric Blood Cancer 2022, e29663. [Google Scholar] [CrossRef] [PubMed]
- Ratko, T.A.; Belinson, S.E.; Brown, H.M.; Noorani, H.Z.; Chopra, R.D.; Marbella, A.; Samson, D.J.; Bonnell, C.J.; Ziegler, K.M.; Aronson, N. Hematopoietic Stem-Cell Transplantation in the Pediatric Population. AHRQ Comparative Effectiveness Reviews; Report No.: 12-EHC018-EF; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2012.
- Kushner, B.H.; Cheung, I.Y.; Modak, S.; Basu, E.M.; Roberts, S.S.; Cheung, N.-K. Humanized 3F8 Anti-GD2 Monoclonal Antibody Dosing with Granulocyte-Macrophage Colony-Stimulating Factor in Pa-tients With Resistant Neuroblastoma: A Phase 1 Clinical Trial. JAMA Oncol. 2018, 4, 1729–1735. [Google Scholar] [CrossRef] [Green Version]
- Mody, R.; Naranjo, A.; Van Ryn, C.; Yu, A.L.; London, W.B.; Shulkin, B.L.; Parisi, M.T.; Servaes, S.-E.-N.; Diccianni, M.B.; Sondel, P.M.; et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): An open-label, randomised, phase 2 trial. Lancet Oncol. 2017, 18, 946–957. [Google Scholar] [CrossRef] [Green Version]
- Kushner, B.H.; Kramer, K.; Cheung, N.-K.V. Phase II trial of the anti-G(D2) monoclonal antibody 3F8 and granulocyte macrophage colony-stimulating factor for neuro-blastoma. J. Clin. Oncol. 2001, 19, 4189–4194. [Google Scholar] [CrossRef]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef] [Green Version]
- Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Yaniv, I.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1617–1629. [Google Scholar] [CrossRef]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; Naranjo, A.; Diccianni, M.B.; Gan, J.; Hank, J.A.; Batova, A.; London, W.B.; Tenney, S.C.; et al. Long-Term Follow-up of a Phase III Study of ch14.18 (Dinutuximab) þ Cytokine Immunotherapy in Children with High-Risk Neuroblastoma: COG Study ANBL0032. Clin. Cancer Res. 2021, 27, 2179–2189. [Google Scholar] [CrossRef]
- Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Ash, S.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Investigation of the Role of Dinutuximab Beta-Based Immunotherapy in the SIOPEN High-Risk Neuroblastoma 1 Trial (HR-NBL1). Cancers 2020, 12, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, N.-K.V.; Cheung, I.Y.; Kushner, B.H.; Ostrovnaya, I.; Kramer, K.; Modak, S. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J. Clin. Oncol. 2012, 30, 3264–3270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushner, B.H.; Ostrovnaya, I.; Cheung, I.Y.; Kuk, D.; Modak, S.; Kramer, K.; Roberts, S.S.; Basu, E.M.; Yataghene, K.; Cheung, N.-K.V. Lack of survival advantage with autologous stem-cell transplantation in high-risk neuroblastoma consolidated by anti-GD2 immunotherapy and isotretinoin. Oncotarget 2016, 7, 4155–4166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushner, B.H.; LaQuaglia, M.P.; Modak, S.; Wolden, S.L.; Basu, E.M.; Roberts, S.S.; Kramer, K.; Yataghene, K.; Cheung, I.Y.; Cheung, N.-K.V. MYCN-amplified stage 2/3 neuroblastoma: Excellent survival in the era of anti-GD2 immunotherapy. Oncotarget 2017, 8, 95293–95302. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.; Castañeda, A.; Flores, M.A.; Santa-María, V.; Garraus, M.; Gorostegui, M.; Simao, M.; Perez-Jaume, S.; Mañe, S. The Role of Autologous Stem-Cell Transplantation in High-Risk Neuroblastoma Consolidated by anti-GD2 Immunothera-py. Results of Two Consecutive Studies. Front. Pharmacol. 2020, 11, 1699. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.; Castañeda, A.; Gorostegui, M.; Santa-María, V.; Garraus, M.; Muñoz, J.P.; Varo, A.; Perez-Jaume, S.; Mañe, S. Naxitamab combined with granulocyte-macrophage colony-stimulating factor as consolidation for high-risk neuroblastoma patients in complete remission. Pediatric Blood Cancer 2021, 68, e29121. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora, J. Autologous Stem-Cell Transplantation for High-Risk Neuroblastoma: Historical and Critical Review. Cancers 2022, 14, 2572. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14112572
Mora J. Autologous Stem-Cell Transplantation for High-Risk Neuroblastoma: Historical and Critical Review. Cancers. 2022; 14(11):2572. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14112572
Chicago/Turabian StyleMora, Jaume. 2022. "Autologous Stem-Cell Transplantation for High-Risk Neuroblastoma: Historical and Critical Review" Cancers 14, no. 11: 2572. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14112572