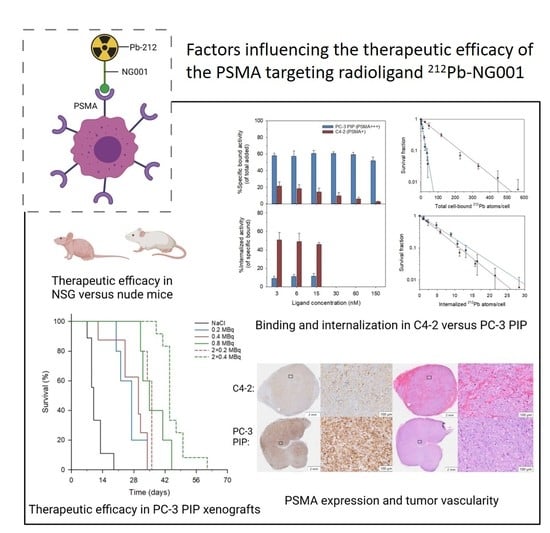
Factors Influencing the Therapeutic Efficacy of the PSMA Targeting Radioligand 212Pb-NG001
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of 212Pb and Activity Measurements
2.2. Radiolabelling of NG001 with 212Pb
2.3. Cell Lines
2.4. Binding and Internalization of 212Pb-NG001 in C4-2 and PC-3 PIP Cells
2.5. Clonogenic Assay
2.6. Animals and Tumor Xenografts
2.7. Immunohistochemical (IHC) and Hematoxylin and Eosin (H&E) Staining
2.8. Biodistribution of 212Pb-NG001 in Mice with PC-3 PIP Xenografts
2.9. Therapeutic Effect of 212Pb-NG001 in Mice with PC-3 PIP and C4-2 Xenografts
2.10. Statistics
3. Results
3.1. Cell Binding, Internalization, and Cytotoxicity of 212Pb-NG001 in C4-2 and PC-3 PIP Cells
3.2. Biodistribution of 212Pb-NG001 in Mice with PC-3 PIP and C4-2 Xenografts
3.3. Therapeutic Effect of 212Pb-NG001 in Mice with PC-3 PIP Xenografts
3.4. PSMA Expression in Xenograft Tumors and Kidneys
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hupe, M.C.; Philippi, C.; Roth, D.; Kumpers, C.; Ribbat-Idel, J.; Becker, F.; Joerg, V.; Duensing, S.; Lubczyk, V.H.; Kirfel, J.; et al. Expression of Prostate-Specific Membrane Antigen (PSMA) on Biopsies Is an Independent Risk Stratifier of Prostate Cancer Patients at Time of Initial Diagnosis. Front. Oncol. 2018, 8, 623. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, A.; Sheehan, B.; Riisnaes, R.; Rodrigues, D.N.; Gurel, B.; Bertan, C.; Ferreira, A.; Lambros, M.B.K.; Seed, G.; Yuan, W.; et al. Prostate-specific Membrane Antigen Heterogeneity and DNA Repair Defects in Prostate Cancer. Eur. Urol. 2019, 76, 469–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, G.L., Jr.; Haley, C.; Beckett, M.L.; Schellhammer, P.F. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol. Oncol. 1995, 1, 18–28. [Google Scholar] [CrossRef]
- Liu, H.; Rajasekaran, A.K.; Moy, P.; Xia, Y.; Kim, S.; Navarro, V.; Rahmati, R.; Bander, N.H. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 1998, 58, 4055–4060. [Google Scholar]
- Rajasekaran, S.A.; Anilkumar, G.; Oshima, E.; Bowie, J.U.; Liu, H.; Heston, W.; Bander, N.H.; Rajasekaran, A.K. A novel cytoplasmic tail MXXXL motif mediates the internalization of prostate-specific membrane antigen. Mol. Biol. Cell 2003, 14, 4835–4845. [Google Scholar] [CrossRef] [Green Version]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benešová, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy of Metastatic Castration-Resistant Prostate Cancer with 177Lu-Labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef] [Green Version]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schäfers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Lawal, I.O.; Bruchertseifer, F.; Vorster, M.; Morgenstern, A.; Sathekge, M.M. Prostate-specific membrane antigen-targeted endoradiotherapy in metastatic prostate cancer. Curr. Opin. Urol. 2020, 30, 98–105. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Dwivedi, S.N.; Bal, C. Radioligand Therapy with (177)Lu-PSMA for Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. AJR Am. J. Roentgenol. 2019, 213, 275–285. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted alpha-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Tripathi, M.; Seth, A.; Bal, C. Efficacy and safety of (225)Ac-PSMA-617 targeted alpha therapy in metastatic castration-resistant Prostate Cancer patients. Theranostics 2020, 10, 9364–9377. [Google Scholar] [CrossRef]
- Feuerecker, B.; Tauber, R.; Knorr, K.; Heck, M.; Beheshti, A.; Seidl, C.; Bruchertseifer, F.; Pickhard, A.; Gafita, A.; Kratochwil, C.; et al. Activity and Adverse Events of Actinium-225-PSMA-617 in Advanced Metastatic Castration-resistant Prostate Cancer After Failure of Lutetium-177-PSMA. Eur. Urol. 2021, 79, 343–350. [Google Scholar] [CrossRef]
- Satapathy, S.; Sood, A.; Das, C.K.; Mittal, B.R. Evolving role of (225)Ac-PSMA radioligand therapy in metastatic castration-resistant prostate cancer-a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2021, 24, 880–890. [Google Scholar] [CrossRef]
- Juzeniene, A.; Stenberg, V.Y.; Bruland, Ø.S.; Larsen, R.H. Preclinical and Clinical Status of PSMA-Targeted Alpha Therapy for Metastatic Castration-Resistant Prostate Cancer. Cancers 2021, 13, 779. [Google Scholar] [CrossRef]
- Stenberg, V.Y.; Larsen, R.H.; Ma, L.W.; Peng, Q.; Juzenas, P.; Bruland, Ø.S.; Juzeniene, A. Evaluation of the PSMA-Binding Ligand (212)Pb-NG001 in Multicellular Tumor Spheroid and Mouse Models of Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 4815. [Google Scholar] [CrossRef]
- Banerjee, S.R.; Minn, I.; Kumar, V.; Josefsson, A.; Lisok, A.; Brummet, M.; Chen, J.; Kiess, A.P.; Baidoo, K.; Brayton, C.; et al. Preclinical Evaluation of (203/212)Pb-Labeled Low-Molecular-Weight Compounds for Targeted Radiopharmaceutical Therapy of Prostate Cancer. J. Nucl. Med. 2020, 61, 80–88. [Google Scholar] [CrossRef]
- Dos Santos, J.C.; Schafer, M.; Bauder-Wust, U.; Lehnert, W.; Leotta, K.; Morgenstern, A.; Kopka, K.; Haberkorn, U.; Mier, W.; Kratochwil, C. Development and dosimetry of (203)Pb/(212)Pb-labelled PSMA ligands: Bringing “the lead” into PSMA-targeted alpha therapy? Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1081–1091. [Google Scholar] [CrossRef] [Green Version]
- Hammer, S.; Hagemann, U.B.; Zitzmann-Kolbe, S.; Larsen, A.; Ellingsen, C.; Geraudie, S.; Grant, D.; Indrevoll, B.; Smeets, R.; von Ahsen, O.; et al. Preclinical Efficacy of a PSMA-Targeted Thorium-227 Conjugate (PSMA-TTC), a Targeted Alpha Therapy for Prostate Cancer. Clin. Cancer Res. 2020, 26, 1985–1996. [Google Scholar] [CrossRef] [Green Version]
- Ferrier, M.G.; Radchenko, V. An Appendix of Radionuclides Used in Targeted Alpha Therapy. J. Med. Imaging Radiat. Sci. 2019, 50, S58–S65. [Google Scholar] [CrossRef] [Green Version]
- Mease, R.C.; Kang, C.; Kumar, V.; Ray, S.; Minn, I.L.; Brummet, M.; Gabrielson, K.; Feng, Y.; Park, A.; Kiess, A.; et al. An improved (211)At-labeled agent for PSMA-targeted alpha therapy. J. Nucl. Med. 2022, 63, 259–267. [Google Scholar] [CrossRef]
- Umbricht, C.A.; Köster, U.; Bernhardt, P.; Gracheva, N.; Johnston, K.; Schibli, R.; van der Meulen, N.P.; Müller, C. Alpha-PET for Prostate Cancer: Preclinical investigation using 149Tb-PSMA-617. Sci. Rep. 2019, 9, 17800. [Google Scholar] [CrossRef] [Green Version]
- Stenberg, V.Y.; Juzeniene, A.; Chen, Q.; Yang, X.; Bruland, O.S.; Larsen, R.H. Preparation of the alpha-emitting PSMA targeted radioligand [(212)Pb]Pb-NG001 for prostate cancer. J. Label. Comp. Radiopharm. 2020, 63, 129–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonnekens, J.; Schottelius, M. “Luke! Luke! Don’t! It’s a trap!”—Spotlight on bias in animal experiments in nuclear oncology. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1024–1026. [Google Scholar] [CrossRef] [Green Version]
- Tschan, V.J.; Borgna, F.; Schibli, R.; Müller, C. Impact of the mouse model and molar amount of injected ligand on the tissue distribution profile of PSMA radioligands. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Benesova, M.; Schafer, M.; Bauder-Wust, U.; Afshar-Oromieh, A.; Kratochwil, C.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2015, 56, 914–920. [Google Scholar] [CrossRef] [Green Version]
- Benesova, M.; Umbricht, C.A.; Schibli, R.; Muller, C. Albumin-Binding PSMA Ligands: Optimization of the Tissue Distribution Profile. Mol. Pharm. 2018, 15, 934–946. [Google Scholar] [CrossRef]
- Kuo, H.T.; Merkens, H.; Zhang, Z.; Uribe, C.F.; Lau, J.; Zhang, C.; Colpo, N.; Lin, K.S.; Benard, F. Enhancing Treatment Efficacy of (177)Lu-PSMA-617 with the Conjugation of an Albumin-Binding Motif: Preclinical Dosimetry and Endoradiotherapy Studies. Mol. Pharm. 2018, 15, 5183–5191. [Google Scholar] [CrossRef]
- Lückerath, K.; Wei, L.; Fendler, W.P.; Evans-Axelsson, S.; Stuparu, A.D.; Slavik, R.; Mona, C.E.; Calais, J.; Rettig, M.; Reiter, R.E.; et al. Preclinical evaluation of PSMA expression in response to androgen receptor blockade for theranostics in prostate cancer. EJNMMI Res. 2018, 8, 96. [Google Scholar] [CrossRef]
- Stuparu, A.D.; Capri, J.R.; Meyer, C.; Le, T.M.; Evans-Axelsson, S.L.; Current, K.; Lennox, M.; Mona, C.E.; Fendler, W.P.; Calais, J.; et al. Mechanisms of Resistance to Prostate-Specific Membrane Antigen-Targeted Radioligand Therapy in a Mouse Model of Prostate Cancer. J. Nucl. Med. 2020, 62, 989–995. [Google Scholar] [CrossRef]
- Chatalic, K.L.; Heskamp, S.; Konijnenberg, M.; Molkenboer-Kuenen, J.D.; Franssen, G.M.; Clahsen-van Groningen, M.C.; Schottelius, M.; Wester, H.J.; van Weerden, W.M.; Boerman, O.C.; et al. Towards Personalized Treatment of Prostate Cancer: PSMA I&T, a Promising Prostate-Specific Membrane Antigen-Targeted Theranostic Agent. Theranostics 2016, 6, 849–861. [Google Scholar] [CrossRef]
- Wang, X.; Ma, D.; Olson, W.C.; Heston, W.D. In vitro and in vivo responses of advanced prostate tumors to PSMA ADC, an auristatin-conjugated antibody to prostate-specific membrane antigen. Mol. Cancer Ther. 2011, 10, 1728–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDevitt, M.R.; Barendswaard, E.; Ma, D.; Lai, L.; Curcio, M.J.; Sgouros, G.; Ballangrud, A.M.; Yang, W.H.; Finn, R.D.; Pellegrini, V.; et al. An alpha-particle emitting antibody ([213Bi]J591) for radioimmunotherapy of prostate cancer. Cancer Res. 2000, 60, 6095–6100. [Google Scholar] [PubMed]
- Fendler, W.P.; Stuparu, A.D.; Evans-Axelsson, S.; Luckerath, K.; Wei, L.; Kim, W.; Poddar, S.; Said, J.; Radu, C.G.; Eiber, M.; et al. Establishing (177)Lu-PSMA-617 Radioligand Therapy in a Syngeneic Model of Murine Prostate Cancer. J. Nucl. Med. 2017, 58, 1786–1792. [Google Scholar] [CrossRef] [Green Version]
- Kiess, A.P.; Minn, I.; Chen, Y.; Hobbs, R.; Sgouros, G.; Mease, R.C.; Pullambhatla, M.; Shen, C.J.; Foss, C.A.; Pomper, M.G. Auger Radiopharmaceutical Therapy Targeting Prostate-Specific Membrane Antigen. J. Nucl. Med. 2015, 56, 1401–1407. [Google Scholar] [CrossRef] [Green Version]
- Current, K.; Meyer, C.; Magyar, C.E.; Mona, C.E.; Almajano, J.; Slavik, R.; Stuparu, A.D.; Cheng, C.; Dawson, D.W.; Radu, C.G.; et al. Investigating PSMA-Targeted Radioligand Therapy Efficacy as a Function of Cellular PSMA Levels and Intratumoral PSMA Heterogeneity. Clin. Cancer Res. 2020, 26, 2946–2955. [Google Scholar] [CrossRef]
- Birindelli, G.; Drobnjakovic, M.; Morath, V.; Steiger, K.; D’Alessandria, C.; Gourni, E.; Afshar-Oromieh, A.; Weber, W.; Rominger, A.; Eiber, M.; et al. Is Hypoxia a Factor Influencing PSMA-Directed Radioligand Therapy?—An In Silico Study on the Role of Chronic Hypoxia in Prostate Cancer. Cancers 2021, 13, 3429. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, W.; Rachagani, S.; Zhou, Z.; Lele, S.M.; Batra, S.K.; Garrison, J.C. Comparative Study of Subcutaneous and Orthotopic Mouse Models of Prostate Cancer: Vascular Perfusion, Vasculature Density, Hypoxic Burden and BB2r-Targeting Efficacy. Sci. Rep. 2019, 9, 11117. [Google Scholar] [CrossRef] [Green Version]
- Kasperzyk, J.L.; Finn, S.P.; Flavin, R.; Fiorentino, M.; Lis, R.; Hendrickson, W.K.; Clinton, S.K.; Sesso, H.D.; Giovannucci, E.L.; Stampfer, M.J.; et al. Prostate-specific membrane antigen protein expression in tumor tissue and risk of lethal prostate cancer. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2354–2363. [Google Scholar] [CrossRef] [Green Version]
- Grant, C.L.; Caromile, L.A.; Ho, V.; Durrani, K.; Rahman, M.M.; Claffey, K.P.; Fong, G.-H.; Shapiro, L.H. Prostate specific membrane antigen (PSMA) regulates angiogenesis independently of VEGF during ocular neovascularization. PLoS ONE 2012, 7, e41285. [Google Scholar] [CrossRef]
- Ngen, E.J.; Chen, Y.; Azad, B.B.; Boinapally, S.; Jacob, D.; Lisok, A.; Shen, C.; Hossain, M.S.; Jin, J.; Bhujwalla, Z.M.; et al. Prostate-specific membrane antigen (PSMA)-targeted photodynamic therapy enhances the delivery of PSMA-targeted magnetic nanoparticles to PSMA-expressing prostate tumors. Nanotheranostics 2021, 5, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.A.; Chang, S.H.; Dvorak, A.M.; Dvorak, H.F. Why are tumor blood vessels abnormal and why is it important to know? Br. J. Cancer 2009, 100, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Piron, S.; Verhoeven, J.; de Coster, E.; Descamps, B.; Kersemans, K.; Pieters, L.; Vral, A.; Vanhove, C.; de Vos, F. Impact of the molar activity and PSMA expression level on [(18)F]AlF-PSMA-11 uptake in prostate cancer. Sci. Rep. 2021, 11, 22623. [Google Scholar] [CrossRef] [PubMed]
- Wurzer, A.; Pollmann, J.; Schmidt, A.; Reich, D.; Wester, H.J.; Notni, J. Molar Activity of Ga-68 Labeled PSMA Inhibitor Conjugates Determines PET Imaging Results. Mol. Pharm. 2018, 15, 4296–4302. [Google Scholar] [CrossRef]
- Luurtsema, G.; Pichler, V.; Bongarzone, S.; Seimbille, Y.; Elsinga, P.; Gee, A.; Vercouillie, J. EANM guideline for harmonisation on molar activity or specific activity of radiopharmaceuticals: Impact on safety and imaging quality. EJNMMI Radiopharm. Chem. 2021, 6, 34. [Google Scholar] [CrossRef]
- Soeda, F.; Watabe, T.; Naka, S.; Liu, Y.; Horitsugi, G.; Neels, O.C.; Kopka, K.; Tatsumi, M.; Shimosegawa, E.; Giesel, F.L.; et al. Impact of (18)F-PSMA-1007 Uptake in Prostate Cancer Using Different Peptide Concentrations: Preclinical PET/CT Study on Mice. J. Nucl. Med. 2019, 60, 1594–1599. [Google Scholar] [CrossRef] [Green Version]
- Kalidindi, T.M.; Lee, S.G.; Jou, K.; Chakraborty, G.; Skafida, M.; Tagawa, S.T.; Bander, N.H.; Schoder, H.; Bodei, L.; Pandit-Taskar, N.; et al. A simple strategy to reduce the salivary gland and kidney uptake of PSMA-targeting small molecule radiopharmaceuticals. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2642–2651. [Google Scholar] [CrossRef]
- Stallons, T.A.R.; Saidi, A.; Tworowska, I.; Delpassand, E.S.; Torgue, J.J. Preclinical Investigation of (212)Pb-DOTAMTATE for Peptide Receptor Radionuclide Therapy in a Neuroendocrine Tumor Model. Mol. Cancer Ther. 2019, 18, 1012–1021. [Google Scholar] [CrossRef] [Green Version]
- Kuik, W.-J.; Kema, I.P.; Brouwers, A.H.; Zijlma, R.; Neumann, K.D.; Dierckx, R.A.J.O.; DiMagno, S.G.; Elsinga, P.H. In Vivo Biodistribution of No-Carrier-Added 6-(18)F-Fluoro-3,4-Dihydroxy-L-Phenylalanine ((18)F-DOPA), Produced by a New Nucleophilic Substitution Approach, Compared with Carrier-Added (18)F-DOPA, Prepared by Conventional Electrophilic Substitution. J. Nucl. Med. 2015, 56, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Eychenne, R.; Chérel, M.; Haddad, F.; Guérard, F.; Gestin, J.F. Overview of the Most Promising Radionuclides for Targeted Alpha Therapy: The “Hopeful Eight”. Pharmaceutics 2021, 13, 906. [Google Scholar] [CrossRef]
- Heyerdahl, H.; Abbas, N.; Brevik, E.M.; Mollatt, C.; Dahle, J. Fractionated therapy of HER2-expressing breast and ovarian cancer xenografts in mice with targeted alpha emitting 227Th-DOTA-p-benzyl-trastuzumab. PLoS ONE 2012, 7, e42345. [Google Scholar] [CrossRef]
- Jurcic, J.; Levy, M.; Park, J.; Ravandi, F.; Perl, A.; Pagel, J.; Smith, B.; Orozco, J.; Estey, E.; Kantarjian, H.; et al. Phase I trial of alpha-particle immunotherapy with 225Ac-lintuzumab and low-dose cytarabine in patients age 60 or older with untreated acute myeloid leukemia. J. Nucl. Med. 2017, 58, 456. [Google Scholar]
- Shultz, L.D.; Ishikawa, F.; Greiner, D.L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 2007, 7, 118–130. [Google Scholar] [CrossRef]
- Budach, W.; Taghian, A.; Freeman, J.; Gioioso, D.; Suit, H.D. Impact of stromal sensitivity on radiation response of tumors. J. Natl. Cancer Inst. 1993, 85, 988–993. [Google Scholar] [CrossRef]
- Ogawa, K.; Boucher, Y.; Kashiwagi, S.; Fukumura, D.; Chen, D.; Gerweck, L.E. Influence of tumor cell and stroma sensitivity on tumor response to radiation. Cancer Res. 2007, 67, 4016–4021. [Google Scholar] [CrossRef] [Green Version]
- Larsen, R.H. Production of Highly Purified 212Pb. Patent WO2021110950A1, June 2010. [Google Scholar]
- Westrom, S.; Generalov, R.; Bonsdorff, T.B.; Larsen, R.H. Preparation of (212)Pb-labeled monoclonal antibody using a novel (224)Ra-based generator solution. Nucl. Med. Biol. 2017, 51, 1–9. [Google Scholar] [CrossRef]
- Napoli, E. Quantification Methods of 224Ra and 212Pb Activity Applied to Characterize Therapeutic Radiopharmaceuticals. Ph.D. Thesis, University of Oslo, Oslo, Norway, 2021. [Google Scholar]
- Larsen, R.H. Lead and Thorium Compounds. U.S. Patent US10377778B2, 13 August 2019. [Google Scholar]
- Li, R.G.; Stenberg, V.Y.; Larsen, R.H. A Novel Experimental Generator for Production of High Purity Lead-212 for Use in Radiopharmaceuticals; Nucligen AS; Oslo University Hospital: Oslo, Norway, 2022; Manuscript submitted for publication. [Google Scholar]
- Napoli, E.; Stenberg, V.Y.; Juzeniene, A.; Hjellum, G.E.; Bruland, Ø.S.; Larsen, R.H. Calibration of sodium iodide detectors and reentrant ionization chambers for (212)Pb activity in different geometries by HPGe activity determined samples. Appl. Radiat. Isot. 2020, 166, 109362. [Google Scholar] [CrossRef]
- Umbricht, C.A.; Benesova, M.; Schmid, R.M.; Turler, A.; Schibli, R.; van der Meulen, N.P.; Muller, C. (44)Sc-PSMA-617 for radiotheragnostics in tandem with (177)Lu-PSMA-617-preclinical investigations in comparison with (68)Ga-PSMA-11 and (68)Ga-PSMA-617. EJNMMI Res. 2017, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Müller, C.; Singh, A.; Umbricht, C.A.; Kulkarni, H.R.; Johnston, K.; Benešová, M.; Senftleben, S.; Müller, D.; Vermeulen, C.; Schibli, R.; et al. Preclinical investigations and first-in-human application of 152Tb-PSMA-617 for PET/CT imaging of prostate cancer. EJNMMI Res. 2019, 9, 68. [Google Scholar] [CrossRef]
- Lee, D.; Li, M.; Bednarz, B.; Schultz, M.K. Modeling Cell and Tumor-Metastasis Dosimetry with the Particle and Heavy Ion Transport Code System (PHITS) Software for Targeted Alpha-Particle Radionuclide Therapy. Radiat. Res. 2018, 190, 236–247. [Google Scholar] [CrossRef]
- Sofou, S. Radionuclide carriers for targeting of cancer. Int. J. Nanomed. 2008, 3, 181–199. [Google Scholar] [CrossRef] [Green Version]
- Boudousq, V.; Bobyk, L.; Busson, M.; Garambois, V.; Jarlier, M.; Charalambatou, P.; Pèlegrin, A.; Paillas, S.; Chouin, N.; Quenet, F.; et al. Comparison between internalizing anti-HER2 mAbs and non-internalizing anti-CEA mAbs in alpha-radioimmunotherapy of small volume peritoneal carcinomatosis using 212Pb. PLoS ONE 2013, 8, e69613. [Google Scholar] [CrossRef] [Green Version]
- Azure, M.T.; Archer, R.D.; Sastry, K.S.; Rao, D.V.; Howell, R.W. Biological effect of lead-212 localized in the nucleus of mammalian cells: Role of recoil energy in the radiotoxicity of internal alpha-particle emitters. Radiat. Res. 1994, 140, 276–283. [Google Scholar] [CrossRef]
- Nikula, T.K.; McDevitt, M.R.; Finn, R.D.; Wu, C.; Kozak, R.W.; Garmestani, K.; Brechbiel, M.W.; Curcio, M.J.; Pippin, C.G.; Tiffany-Jones, L.; et al. Alpha-Emitting Bismuth Cyclohexylbenzyl DTPA Constructs of Recombinant Humanized Anti-CD33 Antibodies: Pharmacokinetics, Bioactivity, Toxicity and Chemistry. J. Nucl. Med. 1999, 40, 166–176. [Google Scholar]
- De Kruijff, R.M.; Wolterbeek, H.T.; Denkova, A.G. A Critical Review of Alpha Radionuclide Therapy-How to Deal with Recoiling Daughters? Pharmaceuticals 2015, 8, 321–336. [Google Scholar] [CrossRef]
- Pouget, J.-P.; Constanzo, J. Revisiting the Radiobiology of Targeted Alpha Therapy. Front. Med. 2021, 8, 692436. [Google Scholar] [CrossRef]
- Pouget, J.-P.; Santoro, L.; Piron, B.; Paillas, S.; Ladjohounlou, R.; Pichard, A.; Poty, S.; Deshayes, E.; Constanzo, J.; Bardiès, M. From the target cell theory to a more integrated view of radiobiology in Targeted radionuclide therapy: The Montpellier group’s experience. Nucl. Med. Biol. 2022, 104–105, 53–64. [Google Scholar] [CrossRef]
- Ljungberg, M. Handbook of Nuclear Medicine and Molecular Imaging for Physicists: Modelling, Dosimetry and Radiation Protection, Volume II; CRC Press: Boca Raton, FL, USA, 2022; pp. 267–273. [Google Scholar]
- Nath, S.; Devi, G.R. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef] [Green Version]
- Meredith, R.F.; Torgue, J.J.; Rozgaja, T.A.; Banaga, E.P.; Bunch, P.W.; Alvarez, R.D.; Straughn, J.M., Jr.; Dobelbower, M.C.; Lowy, A.M. Safety and Outcome Measures of First-in-Human Intraperitoneal α Radioimmunotherapy with 212Pb-TCMC-Trastuzumab. Am. J. Clin. Oncol. 2018, 41, 716–721. [Google Scholar] [CrossRef]
- Delpassand, E.S.; Tworowska, I.; Esfandiari, R.; Torgue, J.; Hurt, J.; Shafie, A.; Núñez, R. Targeted Alpha-Emitter Therapy with (212)Pb-DOTAMTATE for the Treatment of Metastatic SSTR-Expressing Neuroendocrine Tumors: First-in-Human, Dose-Escalation Clinical Trial. J. Nucl. Med. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Marignol, L.; Coffey, M.; Lawler, M.; Hollywood, D. Hypoxia in prostate cancer: A powerful shield against tumour destruction? Cancer Treat. Rev. 2008, 34, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, R.P.J.; Müller, C.; Reneman, S.; Melis, M.L.; Breeman, W.A.P.; de Blois, E.; Bangma, C.H.; Krenning, E.P.; van Weerden, W.M.; de Jong, M. A standardised study to compare prostate cancer targeting efficacy of five radiolabelled bombesin analogues. Eur. J. Nucl. Med. 2010, 37, 1386–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osieka, R.; Houchens, D.P.; Goldin, A.; Johnson, R.K. Chemotherapy of human colon cancer xenografts in athymic nude mice. Cancer 1977, 40, 2640–2650. [Google Scholar] [CrossRef]
- Milross, C.G.; Tucker, S.L.; Mason, K.A.; Hunter, N.R.; Peters, L.J.; Milas, L. The effect of tumor size on necrosis and polarographically measured pO2. Acta Oncol. 1997, 36, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Euhus, D.M.; Hudd, C.; LaRegina, M.C.; Johnson, F.E. Tumor measurement in the nude mouse. J. Surg. Oncol. 1986, 31, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Palm, S.; Bäck, T.; Claesson, I.; Danielsson, A.; Elgqvist, J.; Frost, S.; Hultborn, R.; Jensen, H.; Lindegren, S.; Jacobsson, L. Therapeutic Efficacy of Astatine-211–Labeled Trastuzumab on Radioresistant SKOV-3 Tumors in Nude Mice. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.R.; Lisok, A.; Minn, I.; Josefsson, A.; Kumar, V.; Brummet, M.; Boinapally, S.; Brayton, C.; Mease, R.C.; Sgouros, G.; et al. Preclinical Evaluation of (213)Bi- and (225)Ac-Labeled Low-Molecular-Weight Compounds for Radiopharmaceutical Therapy of Prostate Cancer. J. Nucl. Med. 2021, 62, 980–988. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Fendler, W.P.; Reinhardt, S.; Ilhan, H.; Delker, A.; Böning, G.; Gildehaus, F.J.; Stief, C.; Bartenstein, P.; Gratzke, C.; Lehner, S.; et al. Preliminary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget 2017, 8, 3581–3590. [Google Scholar] [CrossRef] [Green Version]
- De Zanger, R.M.S.; Chan, H.S.; Breeman, W.A.P.; de Blois, E. Maintaining radiochemical purity of [177Lu]Lu-DOTA-PSMA-617 for PRRT by reducing radiolysis. J. Radioanal. Nucl. Chem. 2019, 321, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Mannweiler, S.; Amersdorfer, P.; Trajanoski, S.; Terrett, J.A.; King, D.; Mehes, G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol. Oncol. Res. 2009, 15, 167–172. [Google Scholar] [CrossRef]
- Justice, M.J.; Dhillon, P. Using the mouse to model human disease: Increasing validity and reproducibility. Dis. Model Mech. 2016, 9, 101–103. [Google Scholar] [CrossRef] [Green Version]






| Tumor Model | Treatment Group | Effective Molar Activity (MBq/nmol) | Number of Mice | Median Survival | TI | p-Value Towards Control |
|---|---|---|---|---|---|---|
| PC-3 PIP | Control | 9 | 10 | 1 | ||
| 0.2 MBq | 1.13 | 5 | 27 | 2.7 | 0.006 | |
| 0.4 MBq | 1.26 | 8 | 30 | 3.0 | 0.002 | |
| 0.8 MBq | 1.24 | 5 | 35 | 3.5 | 0.006 | |
| 2 × 0.2 MBq | 1.02 | 5 | 36 | 3.6 | 0.005 | |
| 2 × 0.4 MBq | 1.33 | 12 | 44 | 4.4 | <0.001 | |
| C4-2 | Control 1 * | 7 | 23 | |||
| 0.25 MBq * | 0.5 | 8 | 35 | 1.5 | <0.002 | |
| Control 2 * | 8 | 16.5 | ||||
| 0.3 MBq * | 1.1 | 8 | 38.5 | 2.3 | 0.012 | |
| Control 3 * | 6 | 27 | ||||
| 0.4 MBq * | 2.3 | 6 | 74 | 2.7 | <0.001 | |
| 0.8 MBq | 2.4 | 6 | 72.5 | 2.7 | <0.001 | |
| Control 4 | 7 | 35 | ||||
| 0.85 MBq | 2.1 | 6 | 89.5 | 2.6 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenberg, V.Y.; Tornes, A.J.K.; Nilsen, H.R.; Revheim, M.-E.; Bruland, Ø.S.; Larsen, R.H.; Juzeniene, A. Factors Influencing the Therapeutic Efficacy of the PSMA Targeting Radioligand 212Pb-NG001. Cancers 2022, 14, 2784. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14112784
Stenberg VY, Tornes AJK, Nilsen HR, Revheim M-E, Bruland ØS, Larsen RH, Juzeniene A. Factors Influencing the Therapeutic Efficacy of the PSMA Targeting Radioligand 212Pb-NG001. Cancers. 2022; 14(11):2784. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14112784
Chicago/Turabian StyleStenberg, Vilde Yuli, Anna Julie Kjøl Tornes, Hogne Røed Nilsen, Mona-Elisabeth Revheim, Øyvind Sverre Bruland, Roy Hartvig Larsen, and Asta Juzeniene. 2022. "Factors Influencing the Therapeutic Efficacy of the PSMA Targeting Radioligand 212Pb-NG001" Cancers 14, no. 11: 2784. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14112784