Non-Melanoma Skin Cancer Clearance after Medical Treatment Detected with Noninvasive Skin Imaging: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection Criteria
- BCC or SCC lesions;
- medical therapy;
- monitoring with clinical examination, dermoscopy, RCM and/or OCT, with histopathologic or cyto-diagnostic confirmation of all suspected cases of primary NMSC persistence with noninvasive methodology.
- languages other than English;
- case reports and small case series;
- <1 month follow-up;
- use of off-label treatments;
- lesions with pre-tumoral NMSC diagnoses (actinic keratoses);
- studies or subset of lesions with an unclear number of persistent NMSC cases.
2.2. Data Search
2.3. Study Selection and Data Collection
2.4. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Study Population
3.3. Diagnostic Accuracy
3.4. SCC Persistence Review
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Conforti, C.; Corneli, P.; Harwood, C.; Zalaudek, I. Evolving Role of Systemic Therapies in Non-Melanoma Skin Cancer. Clin. Oncol. (R. Coll. Radiol.) 2019, 31, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.W.; Weinstock, M.A.; Harris, A.R.; Hinckley, M.R.; Feldman, S.R.; Fleischer, A.B.; Coldiron, B.M. Incidence Estimate of Nonmelanoma Skin Cancer in the United States, 2006. Arch. Dermatol. 2010, 146, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Kauvar, A.N.B.; Cronin, T.; Roenigk, R.; Hruza, G.; Bennett, R. Consensus for Nonmelanoma Skin Cancer Treatment: Basal Cell Carcinoma, Including a Cost Analysis of Treatment Methods. Dermatol. Surg. 2015, 41, 550–571. [Google Scholar] [CrossRef] [PubMed]
- Kauvar, A.N.B.; Arpey, C.J.; Hruza, G.; Olbricht, S.M.; Bennett, R. Consensus for Nonmelanoma Skin Cancer Treatment, Part II: Squamous Cell Carcinoma, Including a Cost Analysis of Treatment Methods. Dermatol. Surg. 2015, 41, 1214–1240. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.-H.; Park, J.-M.; Song, M.; Jwa, S.-W.; Kim, H.-S.; Ko, H.-C.; Kim, B.-S.; Kim, M.-B. The Use of Dermatoscopy to Monitor Therapeutic Response of Bowen Disease: A Dermatoscopic Pathological Study. Br. J. Dermatol. 2012, 167, 1382–1385. [Google Scholar] [CrossRef]
- Kim, D.P.; Kus, K.J.B.; Ruiz, E. Basal Cell Carcinoma Review. Hematol. Oncol. Clin. N. Am. 2019, 33, 13–24. [Google Scholar] [CrossRef]
- Sidoroff, A.; Thaler, P. Taking Treatment Decisions in Non-Melanoma Skin Cancer—The Place for Topical Photodynamic Therapy (PDT). Photodiagn. Photodyn. Ther. 2010, 7, 24–32. [Google Scholar] [CrossRef]
- Övermark, M.; Koskenmies, S.; Pitkänen, S. A Retrospective Study of Treatment of Squamous Cell Carcinoma In Situ. Acta Derm.-Venereol. 2016, 96, 64–67. [Google Scholar] [CrossRef] [Green Version]
- Morton, C.; Horn, M.; Leman, J.; Tack, B.; Bedane, C.; Tjioe, M.; Ibbotson, S.; Khemis, A.; Wolf, P. Comparison of Topical Methyl Aminolevulinate Photodynamic Therapy with Cryotherapy or Fluorouracil for Treatment of Squamous Cell Carcinoma in Situ: Results of a Multicenter Randomized Trial. Arch. Dermatol. 2006, 142, 729–735. [Google Scholar] [CrossRef]
- Ondo, A.L.; Kerner, J.D.; Kerner, J.V.; Ondo, I.P.; Trainor, P.; Shanler, S.D. Treatment of Cutaneous Squamous Cell Carcinoma In Situ With Curettage Followed by Topical Imiquimod 5% Cream. Dermatol. Surg. 2021, 47, 609–612. [Google Scholar] [CrossRef]
- Berking, C.; Hauschild, A.; Kölbl, O.; Mast, G.; Gutzmer, R. Basal Cell Carcinoma. Dtsch. Ärzteblatt Int. 2014, 111, 389–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, A.; Savas, J.; Doerfler, L. Nonsurgical Treatments for Nonmelanoma Skin Cancer. Dermatol. Clin. 2019, 37, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Weber, P.; Tschandl, P.; Sinz, C.; Kittler, H. Dermatoscopy of Neoplastic Skin Lesions: Recent Advances, Updates, and Revisions. Curr. Treat. Options Oncol. 2018, 19, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahu, A.; Yélamos, O.; Iftimia, N.; Cordova, M.; Alessi-Fox, C.; Gill, M.; Maguluri, G.; Dusza, S.W.; Navarrete-Dechent, C.; González, S.; et al. Evaluation of a Combined Reflectance Confocal Microscopy–Optical Coherence Tomography Device for Detection and Depth Assessment of Basal Cell Carcinoma. JAMA Dermatol. 2018, 154, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.; Casari, A.; Pepe, P.; Moscarella, E.; Zalaudek, I.; Argenziano, G.; Pellacani, G. Confocal Microscopy Insights into the Treatment and Cellular Immune Response of Basal Cell Carcinoma to Photodynamic Therapy. Dermatology 2012, 225, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Guida, S.; Longhitano, S.; Ardigò, M.; Pampena, R.; Ciardo, S.; Bigi, L.; Mandel, V.D.; Vaschieri, C.; Manfredini, M.; Pezzini, C.; et al. Dermoscopy, Confocal Microscopy and Optical Coherence Tomography Features of Main Inflammatory and Autoimmune Skin Diseases: A Systematic Review. Australas. J. Dermatol. 2022, 63, 15–26. [Google Scholar] [CrossRef]
- Guida, S.; De Pace, B.; Ciardo, S.; Farnetani, F.; Pellacani, G. Non-Invasive Imaging for Skin Cancers—The European Experience. Curr. Dermatol. Rep. 2019, 8, 172–181. [Google Scholar] [CrossRef]
- Guida, S.; Arginelli, F.; Farnetani, F.; Ciardo, S.; Bertoni, L.; Manfredini, M.; Zerbinati, N.; Longo, C.; Pellacani, G. Clinical Applications of In Vivo and Ex Vivo Confocal Microscopy. Appl. Sci. 2021, 11, 1979. [Google Scholar] [CrossRef]
- Fuchs, C.S.K.; Ortner, V.K.; Mogensen, M.; Rossi, A.M.; Pellacani, G.; Welzel, J.; Mosterd, K.; Guitera, P.; Nayahangan, L.J.; Johnsson, V.L.; et al. 2021 International Consensus Statement on Optical Coherence Tomography for Basal Cell Carcinoma: Image Characteristics, Terminology and Educational Needs. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 772–778. [Google Scholar] [CrossRef]
- Ulrich, M.; Kanitakis, J.; González, S.; Lange-Asschenfeldt, S.; Stockfleth, E.; Roewert-Huber, J. Evaluation of Bowen Disease by in Vivo Reflectance Confocal Microscopy. Br. J. Dermatol. 2012, 166, 451–453. [Google Scholar] [CrossRef]
- Shahriari, N.; Grant-Kels, J.M.; Rabinovitz, H.S.; Oliviero, M.; Scope, A. Reflectance Confocal Microscopy Criteria of Pigmented Squamous Cell Carcinoma In Situ. Am. J. Dermatopathol. 2018, 40, 173–179. [Google Scholar] [CrossRef]
- Manfredini, M.; Longo, C.; Ferrari, B.; Piana, S.; Benati, E.; Casari, A.; Pellacani, G.; Moscarella, E. Dermoscopic and Reflectance Confocal Microscopy Features of Cutaneous Squamous Cell Carcinoma. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1828–1833. [Google Scholar] [CrossRef]
- Reggiani, C.; Manfredini, M.; Mandel, V.D.; Farnetani, F.; Ciardo, S.; Bassoli, S.; Casari, A.; Guida, S.; Argenziano, G.; Lallas, A.; et al. Update on Non-Invasive Imaging Techniques in Early Diagnosis of Non-Melanoma Skin Cancer. G. Ital. Dermatol. Venereol. 2015, 150, 393–405. [Google Scholar]
- Cinotti, E.; Tognetti, L.; Cartocci, A.; Lamberti, A.; Gherbassi, S.; Orte Cano, C.; Lenoir, C.; Dejonckheere, G.; Diet, G.; Fontaine, M.; et al. Line-Field Confocal Optical Coherence Tomography for Actinic Keratosis and Squamous Cell Carcinoma: A Descriptive Study. Clin. Exp. Dermatol. 2021, 46, 1530–1541. [Google Scholar] [CrossRef]
- Cameron, M.C.; Lee, E.; Hibler, B.P.; Giordano, C.N.; Barker, C.A.; Mori, S.; Cordova, M.; Nehal, K.S.; Rossi, A.M. Basal Cell Carcinoma: Contemporary Approaches to Diagnosis, Treatment, and Prevention. J. Am. Acad. Dermatol. 2019, 80, 321–339. [Google Scholar] [CrossRef]
- Sahu, A.; Oh, Y.; Peterson, G.; Cordova, M.; Navarrete-Dechent, C.; Gill, M.; Alessi-Fox, C.; Gonzalez, S.; Phillips, W.; Wilson, S.; et al. In Vivo Optical Imaging-Guided Targeted Sampling for Precise Diagnosis and Molecular Pathology. Sci. Rep. 2021, 11, 23124. [Google Scholar] [CrossRef]
- Fargnoli, M.C.; Kostaki, D.; Piccioni, A.; Micantonio, T.; Peris, K. Dermoscopy in the Diagnosis and Management of Non-Melanoma Skin Cancers. Eur. J. Dermatol. 2012, 22, 456–463. [Google Scholar] [CrossRef]
- PRISMA-P Group; Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Venturini, M.; Sala, R.; Gonzàlez, S.; Calzavara-Pinton, P.G. Reflectance Confocal Microscopy Allows In Vivo Real-Time Noninvasive Assessment of the Outcome of Methyl Aminolaevulinate Photodynamic Therapy of Basal Cell Carcinoma: BCC Response to MAL-PDT by In Vivo RCM. Br. J. Dermatol. 2013, 168, 99–105. [Google Scholar] [CrossRef]
- Apalla, Z.; Lallas, A.; Tzellos, T.; Sidiropoulos, T.; Lefaki, I.; Trakatelli, M.; Sotiriou, E.; Lazaridou, E.; Evangelou, G.; Patsatsi, A.; et al. Applicability of Dermoscopy for Evaluation of Patients’ Response to Nonablative Therapies for the Treatment of Superficial Basal Cell Carcinoma. Br. J. Dermatol. 2014, 170, 809–815. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.A.; Themstrup, L.; Nürnberg, B.M.; Jemec, G. Adjunct Use of Optical Coherence Tomography Increases the Detection of Recurrent Basal Cell Carcinoma over Clinical and Dermoscopic Examination Alone. Photodiagn. Photodyn. Ther. 2016, 14, 178–184. [Google Scholar] [CrossRef]
- Niculescu, L.; Bierhoff, E.; Hartmann, D.; Ruzicka, T.; Berking, C.; von Braunmühl, T. Optical Coherence Tomography Imaging of Basal Cell Carcinoma Undergoing Photodynamic Therapy: A Pilot Study. Photodiagn. Photodyn. Ther. 2017, 18, 133–137. [Google Scholar] [CrossRef]
- Couzan, C.; Cinotti, E.; Labeille, B.; Vercherin, P.; Rubegni, P.; Cambazard, F.; Perrot, J.L. Reflectance Confocal Microscopy Identification of Subclinical Basal Cell Carcinomas during and after Vismodegib Treatment. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 763–767. [Google Scholar] [CrossRef]
- Banzhaf, C.A.; Themstrup, L.; Ring, H.C.; Mogensen, M.; Jemec, G.B.E. Optical Coherence Tomography Imaging of Non-Melanoma Skin Cancer Undergoing Imiquimod Therapy. Skin Res. Technol. 2014, 20, 170–176. [Google Scholar] [CrossRef]
- Boone, M.A.L.M.; Suppa, M.; Pellacani, G.; Marneffe, A.; Miyamoto, M.; Alarcon, I.; Ruini, C.; Hofmann-Wellenhof, R.; Malvehy, J.; Jemec, G.B.E.; et al. High-Definition Optical Coherence Tomography Algorithm for Discrimination of Basal Cell Carcinoma from Clinical BCC Imitators and Differentiation between Common Subtypes. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1771–1780. [Google Scholar] [CrossRef]




| Included Studies, Author, Year of Publication | Study Type | Lesion Type | Patients, n | Lesions, n | Lesion Location, n (% of Lesions) | Type of Treatment | Follow-Up, Months | Noninvasive Skin Imaging Tools | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Head and neck | Limbs | Trunk | Genitalia | ||||||||
| Longo 2012 [15] | Pro | BCC | 10 | 12 | 4 (33) | 3 (25) | 5 (42) | 0 | PDT | 18 | Dermoscopy, RCM |
| Venturini 2013 [29] | Pro | BCC | 20 | 20 | 6 (30) | 2 (10) | 12 (60) | 0 | PDT | 3 | Dermoscopy, RCM |
| Apalla 2014 [30] | Pro | BCC | 55 | 98 | 19 (20) | 22 (22) | 57 (58) | 0 | PDT, Imiquimod | 3–12 | Dermoscopy |
| Hussain 2016 [31] | Pro | BCC | 58 | 58 | n.r. | n.r. | n.r. | n.r. | Curettage ± MAL PDT | 1–36 | Dermoscopy, OCT |
| Niculescu 2017 [32] | Retro | BCC | 10 | 25 | 2 (8) | 8 (32) | 15 (60) | 0 | PDT | 6 | OCT |
| Couzan 2018 [33] | Retro | BCC | 8 | 38/94 * | 45 (48) | 24 (25) | 25 (27) | 0 | Vismodegib | 18 | Dermoscopy, RCM |
| Banzhaf 2014 [34] | Pro | BCC | 16 | 16 | n.r. | n.r. | n.r. | n.r. | Imiquimod | 1 | OCT |
| Mun 2012 [5] | Pro | SCC | 23 | 29 | 0 | 16 (55) | 11 (38) | 2 (7) | PDT, Imiquimod | 9 | Dermoscopy |
| Total | 200 | 296/352 | 78 (26) | 35 (12) | 125 (42) | 2 (1) | |||||
| NMSC Persistence Estimates, n (%) 95% Confidence Interval | ||||||
|---|---|---|---|---|---|---|
| Studies | Lesion, n | Histology/Cyto-Diagnostic Confirmation | Clinical Examination | Dermoscopy | OCT | RCM |
| Longo 2012 [15] | 12 | 2 (16.7) 2.1–48.4 | 0 (0.0) 0–26.5 | 0 (0) 0–26.5 | NP | 2 (16.6) 2.1–48.4 |
| Venturini 2013 [29] | 20 | 5 (25.0) 8.7–49.1 | 2 (10.0) 1.2–31.7 | 3 (15) 3.2–37.9 | NP | 5 (25) 8.6–49.1 |
| Apalla 2014 [30] | 98 | 38 (38.8) 29.1–49.1 | 30 (30.6) 21.7–40.7 | 38 (38.7) 29.1–49.1 | NP | NP |
| Banzhaf 2014 [34] | 8 | 0 (0.0) 0–36.9 | 4 (50.0) 15.7–84.3 | NP | 0 (0.0) 0–36.9 | NP |
| Hussain 2016 [31] | 58 | 12 (20.7) 11.2–33.3 | 9 (15.5) 7.3–27.4 | 9 (15.5) 7.3–27.4 | 15 (25.8) 15.2–39 | NP |
| Couzan 2018 [33] | 38 | 20 (52.6) 35.8–69 | 7 (18.4) 7.7–34.3 | 7 (18.4) 7.7–34.3 | NP | 19 (50) 33.4–66.6 |
| Niculescu 2017 [32] | 25 | 7 (28.0) 12.1–49.4 | 6 (24.0) 9.3–45.1 | NP | 7 (28.0) 12.1–49.4 | NP |
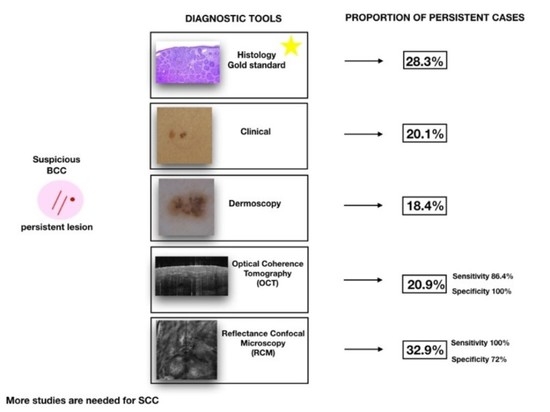
| Total | 259 | 84 (28.3) 17.9–39.9 | 58 (20.1) 12–29.7 | 57 (18.4) 7.7–32.3 | 22 (20.9) 8.7–36.9 | 26 (32.9) 15.3–53.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guida, S.; Alma, A.; Shaniko, K.; Chester, J.; Ciardo, S.; Proietti, I.; Giuffrida, R.; Zalaudek, I.; Manfredini, M.; Longo, C.; et al. Non-Melanoma Skin Cancer Clearance after Medical Treatment Detected with Noninvasive Skin Imaging: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 2836. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14122836
Guida S, Alma A, Shaniko K, Chester J, Ciardo S, Proietti I, Giuffrida R, Zalaudek I, Manfredini M, Longo C, et al. Non-Melanoma Skin Cancer Clearance after Medical Treatment Detected with Noninvasive Skin Imaging: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(12):2836. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14122836
Chicago/Turabian StyleGuida, Stefania, Antonio Alma, Kaleci Shaniko, Johanna Chester, Silvana Ciardo, Ilaria Proietti, Roberta Giuffrida, Iris Zalaudek, Marco Manfredini, Caterina Longo, and et al. 2022. "Non-Melanoma Skin Cancer Clearance after Medical Treatment Detected with Noninvasive Skin Imaging: A Systematic Review and Meta-Analysis" Cancers 14, no. 12: 2836. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14122836