Performances of Functional and Anatomic Imaging Modalities in Succinate Dehydrogenase A-Related Metastatic Pheochromocytoma and Paraganglioma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Selection of Patients
2.2. Patient Cohort and Disease Characteristics
2.3. Imaging Modality Techniques
2.4. Analysis of Data
2.5. Statistical Analysis
3. Results
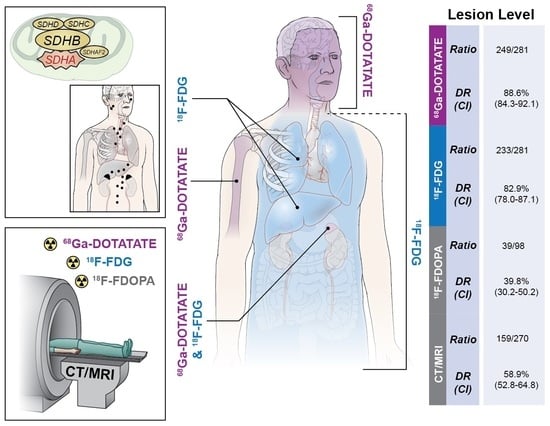
3.1. Lesion Analysis
3.2. Region Analysis
3.3. Patient Analysis
3.4. Summary of Patient, Region, and Lesion Analyses
3.5. Lesions outside the Reference Standard
3.6. Patients with Four Imaging Modalities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jochmanova, I.; Pacak, K. Pheochromocytoma: The First Metabolic Endocrine Cancer. Clin. Cancer Res. 2016, 22, 5001–5011. [Google Scholar] [CrossRef] [PubMed]
- Amar, L.; Pacak, K.; Steichen, O.; Akker, S.A.; Aylwin, S.J.B.; Baudin, E.; Buffet, A.; Burnichon, N.; Clifton-Bligh, R.J.; Dahia, P.L.M.; et al. International consensus on initial screening and follow-up of asymptomatic SDHx mutation carriers. Nat. Rev. Endocrinol. 2021, 17, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Crona, J.; Taieb, D.; Pacak, K. New Perspectives on Pheochromocytoma and Paraganglioma: Toward a Molecular Classification. Endocr. Rev. 2017, 38, 489–515. [Google Scholar] [CrossRef] [PubMed]
- Alrezk, R.; Suarez, A.; Tena, I.; Pacak, K. Update of Pheochromocytoma Syndromes: Genetics, Biochemical Evaluation, and Imaging. Front Endocrinol. 2018, 9, 515. [Google Scholar] [CrossRef] [PubMed]
- Bourgeron, T.; Rustin, P.; Chretien, D.; Birch-Machin, M.; Bourgeois, M.; Viegas-Pequignot, E.; Munnich, A.; Rotig, A. Mutation of a nuclear succinate dehydrogenase gene results in mitochondrial respiratory chain deficiency. Nat. Genet. 1995, 11, 144–149. [Google Scholar] [CrossRef]
- Horvath, R.; Abicht, A.; Holinski-Feder, E.; Laner, A.; Gempel, K.; Prokisch, H.; Lochmuller, H.; Klopstock, T.; Jaksch, M. Leigh syndrome caused by mutations in the flavoprotein (Fp) subunit of succinate dehydrogenase (SDHA). J. Neurol. Neurosurg. Psychiatry 2006, 77, 74–76. [Google Scholar] [CrossRef]
- Burnichon, N.; Briere, J.J.; Libe, R.; Vescovo, L.; Riviere, J.; Tissier, F.; Jouanno, E.; Jeunemaitre, X.; Benit, P.; Tzagoloff, A.; et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum. Mol. Genet. 2010, 19, 3011–3020. [Google Scholar] [CrossRef]
- Jha, A.; de Luna, K.; Balili, C.A.; Millo, C.; Paraiso, C.A.; Ling, A.; Gonzales, M.K.; Viana, B.; Alrezk, R.; Adams, K.T.; et al. Clinical, Diagnostic, and Treatment Characteristics of SDHA-Related Metastatic Pheochromocytoma and Paraganglioma. Front. Oncol. 2019, 9, 53. [Google Scholar] [CrossRef]
- Tufton, N.; Ghelani, R.; Srirangalingam, U.; Kumar, A.V.; Drake, W.M.; Iacovazzo, D.; Skordilis, K.; Berney, D.; Al-Mrayat, M.; Khoo, B.; et al. SDHA mutated paragangliomas may be at high risk of metastasis. Endocr. Relat. Cancer 2017, 24, L43–L49. [Google Scholar] [CrossRef]
- Benn, D.E.; Zhu, Y.; Andrews, K.A.; Wilding, M.; Duncan, E.L.; Dwight, T.; Tothill, R.W.; Burgess, J.; Crook, A.; Gill, A.J.; et al. Bayesian approach to determining penetrance of pathogenic SDH variants. J. Med. Genet. 2018, 55, 729–734. [Google Scholar] [CrossRef]
- Nolting, S.; Bechmann, N.; Taieb, D.; Beuschlein, F.; Fassnacht, M.; Kroiss, M.; Eisenhofer, G.; Grossman, A.; Pacak, K. Personalized management of pheochromocytoma and paraganglioma. Endocr. Rev. 2021, 43, 199–239. [Google Scholar] [CrossRef] [PubMed]
- Bausch, B.; Schiavi, F.; Ni, Y.; Welander, J.; Patocs, A.; Ngeow, J.; Wellner, U.; Malinoc, A.; Taschin, E.; Barbon, G.; et al. Clinical Characterization of the Pheochromocytoma and Paraganglioma Susceptibility Genes SDHA, TMEM127, MAX, and SDHAF2 for Gene-Informed Prevention. JAMA Oncol. 2017, 3, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Taieb, D.; Hicks, R.J.; Hindie, E.; Guillet, B.A.; Avram, A.; Ghedini, P.; Timmers, H.J.; Scott, A.T.; Elojeimy, S.; Rubello, D.; et al. European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2112–2137. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/clinvar/variation/VCV000472322.8 (accessed on 27 July 2022).
- El-Rabadi, K.; Weber, M.; Mayerhofer, M.; Nakuz, T.; Scherer, T.; Mitterhauser, M.; Dudczak, R.; Hacker, M.; Karanikas, G. Clinical Value of 18F-fluorodihydroxyphenylalanine Positron Emission Tomography/Contrast-enhanced Computed Tomography (18F-DOPA PET/CT) in Patients with Suspected Paraganglioma. Anticancer Res. 2016, 36, 4187–4193. [Google Scholar] [PubMed]
- Carrasquillo, J.A.; Chen, C.C.; Jha, A.; Ling, A.; Lin, F.I.; Pryma, D.A.; Pacak, K. Imaging of Pheochromocytoma and Paraganglioma. J. Nucl. Med. 2021, 62, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Belhocine, T.; Spaepen, K.; Dusart, M.; Castaigne, C.; Muylle, K.; Bourgeois, P.; Bourgeois, D.; Dierickx, L.; Flamen, P. 18FDG PET in oncology: The best and the worst (Review). Int. J. Oncol. 2006, 28, 1249–1261. [Google Scholar] [CrossRef]
- Ayala-Ramirez, M.; Palmer, J.L.; Hofmann, M.C.; de la Cruz, M.; Moon, B.S.; Waguespack, S.G.; Habra, M.A.; Jimenez, C. Bone metastases and skeletal-related events in patients with malignant pheochromocytoma and sympathetic paraganglioma. J. Clin. Endocrinol. Metab 2013, 98, 1492–1497. [Google Scholar] [CrossRef]
- Ilanchezhian, M.; Jha, A.; Pacak, K.; Del Rivero, J. Emerging Treatments for Advanced/Metastatic Pheochromocytoma and Paraganglioma. Curr. Treat. Options Oncol. 2020, 21, 85. [Google Scholar] [CrossRef]
- Pacak, K.; Tella, S.H. Pheochromocytoma and Paraganglioma. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dungan, K., Grossman, A., Hershman, J.M., Hofland, H.J., Kaltsas, G., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Jha, A.; Patel, M.; Saboury, B.; Millo, C.; Ling, A.; Shah, R.; Chen, C.; Meuter, L.; Talvacchio, S.; Knue, M.; et al. Superiority of 68Ga-DOTATATE PET/CT compared to 18F-FDG PET/CT and MRI of the spine in the detection of spinal bone metastases in metastatic pheochromocytoma and/or paraganglioma. J. Nucl. Med. 2020, 61, 125. [Google Scholar]
- Van Loon, K.; Zhang, L.; Keiser, J.; Carrasco, C.; Glass, K.; Ramirez, M.T.; Bobiak, S.; Nakakura, E.K.; Venook, A.P.; Shah, M.H.; et al. Bone metastases and skeletal-related events from neuroendocrine tumors. Endocr. Connect. 2015, 4, 9–17. [Google Scholar] [CrossRef]
- Hamidi, O.; Young, W.F., Jr.; Iniguez-Ariza, N.M.; Kittah, N.E.; Gruber, L.; Bancos, C.; Tamhane, S.; Bancos, I. Malignant Pheochromocytoma and Paraganglioma: 272 Patients Over 55 Years. J. Clin. Endocrinol. Metab. 2017, 102, 3296–3305. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Taieb, D.; Jha, A.; Treglia, G.; Pacak, K. Molecular imaging and radionuclide therapy of pheochromocytoma and paraganglioma in the era of genomic characterization of disease subgroups. Endocr. Relat. Cancer 2019, 26, R627–R652. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.I.; Jha, A.; van Berkel, A.; Wild, D.; Janssen, I.; Millo, C.M.; Janssen, M.J.R.; Gonzales, M.K.; Timmers, H.; Pacak, K. Eruption of Metastatic Paraganglioma After Successful Therapy with (177)Lu/(90)Y-DOTATOC and (177)Lu-DOTATATE. Nucl. Med. Mol. Imaging 2019, 53, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Korpershoek, E.; Favier, J.; Gaal, J.; Burnichon, N.; van Gessel, B.; Oudijk, L.; Badoual, C.; Gadessaud, N.; Venisse, A.; Bayley, J.P.; et al. SDHA immunohistochemistry detects germline SDHA gene mutations in apparently sporadic paragangliomas and pheochromocytomas. J. Clin. Endocrinol. Metab. 2011, 96, E1472–E1476. [Google Scholar] [CrossRef] [PubMed]
- van der Tuin, K.; Mensenkamp, A.R.; Tops, C.M.J.; Corssmit, E.P.M.; Dinjens, W.N.; van de Horst-Schrivers, A.N.A.; Jansen, J.C.; de Jong, M.M.; Kunst, H.P.M.; Kusters, B.; et al. Clinical Aspects of SDHA-Related Pheochromocytoma and Paraganglioma: A Nationwide Study. J. Clin. Endocrinol. Metab. 2018, 103, 438–445. [Google Scholar] [CrossRef]

| PT ID i | Sex | SDHA Mutation | Age (d) ii | Age (s) iii | Primary Tumor | Biochemical Phenotype/s iv | Time to Metastasis | Metastatic Location/s | Treatment/s | Ki-67 v | Deceased |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | f | c.91C>T (p.Arg31*) | 11 | 23 | Left Vagale PGL | None | meta, 12 mo | Bones, Neck, Abdomen, Lung | Surgery of Primary, Surgery of Recurrence | Not available | No |
| 2 | m | c.91C>T (p.Arg31*) | 57 | 61 | Paraaortic PGL and Left Carotid Body PGL | ADR, DA | meta, 7 mo | Neck and Mediastinum | Surgery | Not available | No |
| 3 | f | c.1534C>T (p.Arg512*) | 53 | 63 | Paraaortic PGL | NA, DA | syn | Bone, Neck, Mediastinum, Abdomen, Pelvis | Partial resection of Primary; SSA; 90Y-DOTATOC; 177Lu-DOTATOC; CVD; bortezomib and clofarabine; combination capecitabine and TMZ | 15% in focal areas of periaortic PGL | Yes |
| 4 | m | c.91C>T (p.Arg31*) | 20 | 23 | Aortocaval PGL | None | syn, 2 mo vi | Bones and Abdomen | Surgery | Not available | No |
| 5 | m | c.91C>T (p.Arg31*) | 14 | 16 | Paracaval PGL | ADR, NA | syn | Bone | Surgery of Primary, 90Y-DOTATOC, SSA, ONC201 | 3.5% in PGL biopsy | No |
| 6 | m | c.1334C>T (p.S445L) VUS | 53 | 59 | Mediastinal PGL | NA, DA | meta, 48 mo | Bones and Mediastinum | Surgery, SSA, TMZ | 15–20% in T10 met | No |
| 7 | m | c.91C>T (p.Arg31*) | 56 | 67 | Left Adrenal PHEO | ADR, NA, DA | meta, 120 mo | Bones, Lung, Liver, Neck | Surgery of Primary, EBRT, 123I-MIBG, CVD | Not available | Yes |
| 8 | f | 5′UTR_3′ UTRdel | 29 | 33 | Porta Hepatis/Right Adrenal PPGL | NA | meta, 20 mo | Bones, Mediastinum, Lungs | Surgery of Primary, Surgery of Recurrence, EBRT, 177Lu-DOTATATE | 20–30% in T7 epidural Met | Yes |
| 9 | m | c.91C>T (p.Arg31*) | 44 | 45 | Paraaortic PGL | None | syn | Bones, Lung, Mediastinum, Abdomen | Surgical decompression of Spine Met, 123I-MIBG, TMZ | 10–15% Primary | Yes |
| 10 | f | c.91C>T (p.Arg31*) | 46 | 54 | Aortocaval PGL | ADR, NA | meta, 78 mo | Bone, Mediastinum, Liver, Abdomen, Pelvis, Neck | Surgery of Primary, EBRT, 177Lu-DOTATATE, CVD, Liver embolization, Liver trisegmentectomy | >20% in Liver Mets | Yes |
| 11 | f | c.91C>T (p.Arg31*) | 16 | 17 | Mediastinal PGL | None | meta, 7 mo | Bone | Resection of Primary, resection of recurrent bed | Not available | No |
| 68Ga-DOTATATE | 18F-FDG | 18F-FDOPA | CT/MRI | |
|---|---|---|---|---|
| Total Lesions | 249/281 88.6 (84.3–92.1) | 233/281 82.9 (78.0–87.1) | 39/102 39.8 (30.2–50.2) | 157/270 58.2 (52.0–64.1) |
| Primary Lesions | 9/10 90.0 (55.5–99.8) | 9/10 90.0 (55.5–99.8) | 3/7 42.9 (9.9–81.6) | 7/10 70.0 (34.8–93.3) |
| Mediastinum | 1/1 | 1/1 | - vii | 0/1 |
| Adrenal | 1/1 | 1/1 | 0/1 | 1/1 |
| Abdomen/Pelvis | 5/6 | 6/6 | 2/5 | 6/6 |
| Head/Neck | 2/2 | ½ | 1/1 | 0/2 |
| Metastatic Lesions | 240/271 88.6 (84.2–92.1) | 225/271 83.0 (78.0–87.3) | 36/95 37.9 (28.1–48.4) | 150/260 57.7 (51.4–63.8) |
| Bone | 208/223 | 188/223 | 27/64 | 110/212 |
| Lungs | 11/19 | 15/19 | 3/10 | 19/19 |
| Mediastinum | 4/7 | 5/7 | 3/7 | 4/7 |
| Adrenal | 1/1 | 1/1 | 0/1 | 1/1 |
| Liver | 4/7 | 7/7 | 0/4 | 7/7 |
| Abdomen/Pelvis | 6/7 | 6/7 | 2/5 | 4/7 |
| Head/Neck | 6/7 | 3/7 | ¼ | 5/7 |
| 68Ga-DOTATATE | 18F-FDG | 18F-FDOPA | CT/MRI | |
|---|---|---|---|---|
| All Regions | 32/36 | 32/36 | 14/24 | 30/36 |
| Bone | 9/10 | 10/10 | 5/6 | 8/10 |
| Lungs | 4/4 | 4/4 | 1/2 | 4/4 |
| Mediastinum | 5/6 | 4/6 | 3/5 | 3/6 |
| Liver | 2/2 | 2/2 | 0/1 | 2/2 |
| Adrenal | 2/2 | 2/2 | 0/2 | 2/2 |
| Abdomen/Pelvis | 6/7 | 7/7 | 3/5 | 7/7 |
| Head/Neck | 4/5 | 3/5 | 2/3 | 4/5 |
| 68Ga-DOTATATE | 18F-FDG | 18F-FDOPA | CT/MRI | |||||
|---|---|---|---|---|---|---|---|---|
| Ratio | DR (CI) | Ratio | DR (CI) | Ratio | DR (CI) | Ratio | DR (CI) | |
| Patient Level | 10/11 | 90.9% (58.7–99.8) | 10/11 | 90.9% (58.7–99.8) | 7/7 | 100% (59.0–100.0) | 9/11 | 81.8% (48.2–97.7) |
| Region Level | 32/36 | 88.9% (73.9–96.9) | 32/36 | 88.9% (73.9–96.9) | 14/24 | 58.3% (36.6–77.9) | 30/36 | 83.3% (67.2–93.6) |
| Lesion Level | 249/281 | 88.6% (84.3–92.1) | 233/281 | 82.9% (78.0–87.1) | 39/98 | 39.8% (30.2–50.2) | 159/270 | 58.9% (52.8–64.8) |
| 68Ga-DOTATATE | 18F-FDG | 18F-FDOPA | CT/MRI | |
|---|---|---|---|---|
| Total Lesions | 80/102 78.4 (69.2–90.0) | 81/102 79.4 (70.3–86.8) | 39/102 39.8 (30.2–50.2) | 55/102 53.9 (43.8–63.8) |
| Primary Lesions | 6/7 85.7 (42.1–99.6) | 6/7 85.7 (42.1–99.6) | 3/7 42.9 (9.9–81.6) | 6/7 85.7 (42.1–99.6) |
| Metastatic Lesions | 74/95 77.9 (68.2–85.8) | 75/95 79.0 (69.4–86.6) | 36/95 37.9 (28.1–48.4) | 49/95 51.6 (41.1–62.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, M.; Jha, A.; Ling, A.; Chen, C.C.; Millo, C.; Kuo, M.J.M.; Nazari, M.A.; Talvacchio, S.; Charles, K.; Miettinen, M.; et al. Performances of Functional and Anatomic Imaging Modalities in Succinate Dehydrogenase A-Related Metastatic Pheochromocytoma and Paraganglioma. Cancers 2022, 14, 3886. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14163886
Patel M, Jha A, Ling A, Chen CC, Millo C, Kuo MJM, Nazari MA, Talvacchio S, Charles K, Miettinen M, et al. Performances of Functional and Anatomic Imaging Modalities in Succinate Dehydrogenase A-Related Metastatic Pheochromocytoma and Paraganglioma. Cancers. 2022; 14(16):3886. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14163886
Chicago/Turabian StylePatel, Mayank, Abhishek Jha, Alexander Ling, Clara C. Chen, Corina Millo, Mickey J. M. Kuo, Matthew A. Nazari, Sara Talvacchio, Kailah Charles, Markku Miettinen, and et al. 2022. "Performances of Functional and Anatomic Imaging Modalities in Succinate Dehydrogenase A-Related Metastatic Pheochromocytoma and Paraganglioma" Cancers 14, no. 16: 3886. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14163886