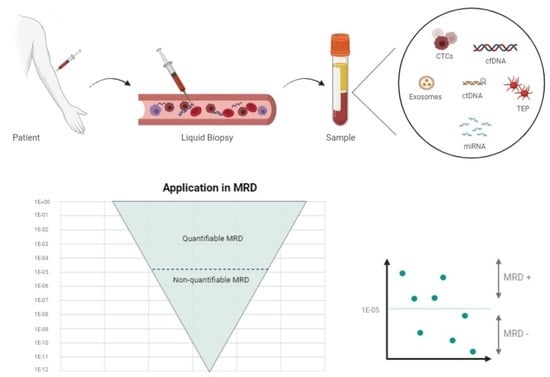
The Minimal Residual Disease Using Liquid Biopsies in Hematological Malignancies
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Liquid Biopsy Components
- Circulating tumor cells (CTCs): CTCs come from tumors as an early step in blood-borne metastasis. CTCs are transient in blood, with a half-life of 1–2.4 h and are presented in a low abundance in most patients. Various techniques have been defined to isolate and analyze CTCs. These cells can even be used to establish cell line models to carry out therapeutic studies [4]. In hematological malignancies, the analysis of CTCs is possible in acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL), myelodysplastic syndromes (MDSs), myeloproliferative neoplasms (MPNs), multiple myelomas (MM), and some lymphomas, such as mantle cell lymphoma (MCL), follicular lymphoma (FL), marginal zone lymphoma, small lymphocytic lymphoma, and a subset of Burkitt lymphoma. In contrast, diffuse large B-cell lymphoma (DLBCL) and classical Hodgkin lymphoma (cHL) do not typically harbor CTCs [5];
- RNA: MicroRNAs (miRNAs) are a class of small molecules of 19–24 nucleotides in length, and they are the most abundant RNA molecules in the blood; they can be carried in the exosomes or TEPs. They have a high stability and play an important role in tumor growth and treatment resistance [6];
- Tumor-educated platelets (TEP): Platelets are circulating anucleated fragments originating from megakaryocytes in the bone marrow, and they participate in hemostasis and the initiation of wound healing. However, they also have a role in systemic and local responses to tumor growth, as tumor cells alter the RNA profile of these platelets. In addition, TEPs can ingest the circulating mRNA released by tumor cells or solubilized tumor-associated proteins [7]. These interactions may signify a potential for cancer diagnosis or monitoring [8];
- Exosomes: Exosomes are a type of extracellular vesicle of endocytic origin, ranging in size between 30 and 100 nm; they are detectable in the blood of patients with some types of cancer, and they carry proteins and nucleic acids. They are analyzed through their RNA content [9]. For example, the ability of HL-specific exosomal microRNAs (miRNAs) to inform a treatment response in HL has been studied. The authors found that the specificity and sensitivity of exosomal miRNAs are superior to protein-bound miRNAs, with regard to HL detection [10];
- DNA: The existence of cell-free DNA (cfDNA) was first described in 1948, and some studies have reported a higher amount of cfDNA in patients with cancer [11,12,13]. Initially, some reports have identified apoptosis, necrosis, or both, as the main source of cfDNA. During apoptosis, the chromosomes are trimmed by DNases into multiple nucleosomal units of 180 bp that are released into the blood stream. These fragments “circulate” for one to three hours before they are ingested by phagocytes and other cells, whereas the cfDNA is completely digested into nucleotides by lysosomes [14]. Necrosis causes the random nonspecific and incomplete digestion of DNA. Some studies have suggested that DNA may be released by living cells [15,16,17]. This review is focused on circulating tumor DNA (ctDNA) and cfDNA, and there are differences between these concepts. cfDNA fragments are longer, with a size range of 160–180 bp, indicating a caspase-dependent apoptotic cleavage, while ctDNA fragments range between 90 and 150 bp, and others range between 250 and 320 bp [18]. The proportion of ctDNA in cfDNA varies, and the most-used methods to detect cfDNA are the real-time quantitative polymerase chain reaction (qRT-PCR) and next-generation sequencing (NGS) [19,20]. Liquid biopsies, by the quantification of cfDNA, can be used for all types of lymphomas, because the plasma from peripheral blood (PB) regularly contains low levels of detectable lymphoma-derived ctDNA, as well as in myeloid pathology (AML, MDS, and MPN) or other lymphoid pathology (MM and ALL) [21]. Therefore, the ctDNA-based liquid biopsy has emerged as a platform to genotype these tumors;
- ○
- dPCR-based methods have a high sensitivity (0.01%) (better than real-time quantitative PCR), but they can only detect a few alterations simultaneously, and they must be optimized for each mutation. In this technique, thousands of individual reactions are analyzed with each sample, calculating whether a PCR end-point is reached, and evaluating the absolute number of molecules in the sample. It is useful in liquid biopsies because it is a precise and sensitive technique, detecting low-abundance targets. [24];
- ○
- In NGS, millions of DNA fragments are generated in a single sequencing process; enrichment can be performed to select the areas of interest. Finally, a massive and parallel sequencing is carried out. NGS-based methods can detect multiple alterations simultaneously. Initially, they had an insufficient sensitivity (>1%), but various groups have been developing methods that allow for a greater sensitivity. Indeed, some of them have tried to use the deep sequencing of a limited number of amplicons for the commonly mutated genes in cancer. The technique, called CAncer Personalized Profiling by deep Sequencing (CAPP-Seq), can detect all major classes of mutations (single nucleotide variants, indels, rearrangements, and copy number alterations). Capture-based NGS methods enrich genomic regions, before sequencing, by a hybridization of target regions to antisense oligonucleotides. With this technique, large portions of the genome can be examined [25].
1.2. Minimal Residual Disease Using Liquid Biopsy
| Method | APL | AML | MDS | MPN | CML |
|---|---|---|---|---|---|
| Image Methods | No | No | No | Yes | No |
| CT or PET/CT MRI | Spleen measurement by CT or MRI (clinical trials) | ||||
| Histologic/morphologic methods | Yes, BM or PB, 10−2 | Yes, BM, 10−2 | Yes, BM, 10−2 | Yes, BM, 10−2 (clinical trials) | No |
| MFC methods | No | Yes, BM, 10−4 or 10−5 | Yes, BM, 10−4 or 10−5 | No recommendations | No |
| Molecular methods | Yes, RQ-PCR (PML/RARA), BM, 10−5 | RQ-PCR *, BM or PB, 10−6 | No recommendations | No recommendations | Yes, PB (BCR/ABL1), 10−5 |
| NGS methods | No | NGS **, BM, 10−6 | Investigational use (clinical trials) | Investigational use (clinical trials) | No |
| Timing of MRD assessment | Post-induction time and PCR every 3 m for 2 years | Upon completion of the initial induction, additional time points should be guided according to the regimen used before allogeneic transplantation | No recommendations | Only in clinical trials *** | PCR every 3 m for one year, then every 6 m |
| References | [33,34] | [34] | [35] | [36,37,38] | [39] |
| Method | ALL | DLBCL | FL | HL | CLL | MM |
|---|---|---|---|---|---|---|
| Image Methods | No | Yes | Yes | Yes | Yes | Yes |
| CT or PET/CT MRI | PET-TAC scan or CT scan contrast | PET-TAC scan or CT scan contrast | CR includes PET negative within 3 m posttreatment. Consider body CT with contrast no more often than every 6 m for the first 2 y following completion of therapy | Lymphoid nodes, spleen, and liver evaluation by CT | PET/CT | |
| Histologic/morphologic methods | Yes, BM, 10−2 | BM biopsy (optional) | BM biopsy (optional) | No, unless there is BM involvement at diagnosis | Yes, BM, 10−2 | Yes, BM with FISH, 10−2 |
| MFC methods | Yes, BM, 10−4 | No | No | No | Yes, BM or PB, 10−5 | Yes, BM, 10−5 |
| Molecular methods | RQ-PCR, BM, 10−6 | No | No | No | ASO-PCR, BM or PB, 10−4 | No |
| NGS methods | NGS Igs-TCR, BM, 10−6 | NGS liquid biopsy, investigational use | NGS liquid biopsy, investigational use | Investigational use | NGS Igs, BM or PB, 10−6 | NGS Igs, BM, 10−6 |
| Timing of MRD assessment | Upon completion of initial induction and additional time points; should be guided according to the regimen used | Post-third cycle and every 3 m | Post-third cycle every 3–6 m for 5 years | Post-third cycle. Additional time points should be guided according to the regimen used | Post-third cycle every 3–6 m for 5 years | Post-third cycle. Consider body CT with contrast no more often than every 6 m for the first 2 y following completion of therapy |
| References | [40] | [41] | [41] | [42] | [43] | [44] |
2. Methods
3. Myeloid Malignancies
3.1. Acute Myeloid Leukemia
| Target | Methods | Cohort Size/Disease Stage | Evidence: Key Points | Application | Reference |
|---|---|---|---|---|---|
| IDH1 and IDH2 genes (R140 and R172 mutations) | Sanger, ddPCR, NGS, and qPCR | n = 60 Diagnosis |
| MRD | Grassi et al., 2020 [53] |
| CEBPA mutations and blast cells | RT-qPCR | n = 4 (n = 3 diagnostic, n = 1 relapse) |
| Concordance | Smith et al., 2006 [56] |
| NPM1 mutations | Flow cytometry, RT-qPCR | n = 15 patients n = 45 MRD samples Diagnosis and follow-up |
| MRD | Pettersson et al., 2016 [51] |
| Somatic mutations | ddPCR, RT-qPCR | n = 41 patients with AML-M1/M2 n = 20 healthy volunteers |
| Concordance | Handschuh et al., 2017 [52] |
| Residual leukemic cells | Flow cytometry | n = 135 patients with de novo AML (100 achieving CR after intensive chemotherapy) |
| MRD Response assessment | Buccisano et al., 2006 [55] |
| Somatic mutation | NGS | n = 22 (after remission) post-treatment |
| Concordance MRD | Short et al., 2020 [67] |
| Driver mutations | NGS dPCR | n = 53 (n = 37 AML, n = 14 MDS) (after post-alloSCT) Diagnosis and post-treatment |
| Concordance MRD Response assessment | Nakamura et al., 2019 [68] |
| Somatic mutation | DDO-ddPCR dPCR qPCR NGS | n = 57 samples (cfDNA), n = 28 (PB), n = 53 (BM) Post-treatment |
| Concordance MRD | Rausch et al., 2021 [69] |
| Leukemic cells | Flow cytometry | n = 50 patients with de novo AML |
| Concordance MRD | Maurillo et al., 2007 [54] |
| Primitive blast (CD34+/CD117+/CD133+) | Flow cytometry | n = 114 patients (205 paired BM and PB samples) Diagnosis and post-treatment |
| Concordance MRD Response assessment | Zeijlemaker et al., 2016 [59] |
3.2. Myelodysplastic Syndromes
| Target | Methods | Cohort Size/Disease Stage | Evidence: Key Points | Application | Ref. |
|---|---|---|---|---|---|
| Somatic mutations | NGS (55 genes) | n = 12 patients 75 samples at follow-up |
| MRD Concordance | Yeh et al., 2017 [73] |
| Somatic mutations | NGS diagnostic (37 genes) ddPCR for follow-up | n = 51 (n = 15 novo AML, n = 22 secondary AML, n = 14 MDS) LOD (NGS) = 0.04% LOD (ddPCR) = 0.1–0.01% |
| Concordance MRD | Nakamura et al., 2019 [68] |
| Somatic mutations (CHIP quantification) | NGS (200 genes) | n = 25 AA patients, n = 27 MDS patients, n = 107 healthy controls LOD = 0.96% |
| Concordance | Gutierrez-Rodrigues et al., 2021 [74] |
3.3. Myeloproliferative Neoplasms
4. Lymphoid Malignancies
4.1. Acute Lymphoblastic Leukemia
4.2. Lymphomas and Chronic Lymphocytic Leukemia
4.2.1. Diffuse Large B-Cell Lymphoma
4.2.2. Mantle Cell Lymphoma
4.2.3. Follicular Lymphoma
4.2.4. Primary Central Nervous System Lymphoma
4.2.5. T-Cell Lymphomas
4.2.6. Hodgkin Lymphoma
4.2.7. Chronic Lymphocytic Leukemia
| Target | Methods | Cohort Size/Disease Stage | Evidence: Key Points | Application | Ref. |
|---|---|---|---|---|---|
| β-globin gene | qPCR | n = 142 DLBCL, n = 63 FL, n = 24 MCL, n = 10 HL, n = 45 |
| Concordance Prognosis | Hohaus et al., 2009 [21] |
| IgH gene rearrangements | Locus-specific primer sets por IgH and IgK | n = 17 DLBCL, n = 15 MLBCL, n = 2 Diagnosis and post-treatment |
| Response assessment Prognosis | Armand et al., 2013 [97] |
| IgH/TCR rearrangements | RQ-PCRNGS | n = 68 B-NHL, n = 37 T-NHL, n = 10 HL, n = 5 CLL, n = 16 Pre- and post-HSCT |
| Prognosis MRD | Herrera et al., 2016 [100] |
| Somatic gene | Targeted NGS | n = 32 B-NHL, n = 18 NK or T-NHL, n = 9 Other, n = 5 |
| Concordance | Shin et al., 2019 [110] |
| APP gene | RT-PCR | n = 174 DLBCL, n = 98 HL, n = 18 TCL, n = 9 NK/T cell lymphoma, n = 21 Other B-NHL, n = 28 Diagnosis |
| Prognosis | Li et al., 2017 [102] |
| IgH gene rearrangements | NGS (ctDNA) and PCR vs. CT | DLBCL, n = 126 Diagnosis and post-treatment |
| Response assessment Staging | Roschewski et al., 2015 [99] |
| IgH gene rearrangements | Ig-HTS vs. PET/CT | DLBCL, n = 75 Diagnosis or recurrence |
| Surveillance after complete remission | Kurtz et al., 2015 [98] |
| Somatic mutations | CAPP-seq Ultra-deep targeted NGS | DLBCL, n = 30 Diagnosis and post-treatment |
| Response assessment | Rossi et al., 2017 [103] |
| V(D)J rearrangements | NGS | DLBCL, n = 6 Pre- and post-CAR-T-cell therapy |
| MRD Concordance | Hossain et al., 2019 [112] |
| L1PA2 | qPCR | DLBCL, n = 40 (and 38 controls) Diagnosis |
| Prognosis | Eskandari et al., 2019 [94] |
| Somatic gene | CAPP-seq | DLBCL, n = 217 Diagnosis, relapse, or recurrence |
| Prognosis | Kurtz et al., 2018 [104] |
| Somatic gene | Targeted NGS | DLBCL, n = 79 Diagnosis and post-treatment |
| Concordance Prognosis | Rivas-Delgado et al., 2021 [105] |
| Ig heavy and light chains and CCND1 and BCL2 genes | NGS | MCL, n = 53 Diagnosis and post-treatment |
| Concordance Prognosis | Lakhotia et al., 2018 [117] |
| V(D)J gene | NGS | FL, n = 133 Diagnosis and post-treatment |
| Concordance Prognosis | Sarkozy et al., 2017 [120] |
| IgH gene rearrangements | ddPCR | FL, n = 133 Diagnosis and post-treatment |
| Concordance Prognosis | Delfau-Larue et al., 2018 [121] |
| Somatic mutations | NGS | FL, n = 27 Diagnosis and post-treatment |
| Concordance Follow-up | Jimenez-Ubieto et al., 2020 [122] |
| MYD88 gene | ddPCR TGS | PCNSL, n = 14 Diagnosis and post-treatment |
| Concordance Follow-up | Hattori et al., 2018 [124] |
| MYD88 gene | ddPCR | PCNSL, n = 29 Diagnosis |
| Concordance | Hiemcke-Jiwa et al., 2019 [126] |
| MYD88 gene | ddPCR | PCNSL, n = 11 Diagnosis and relapse |
| Concordance | Rimelen et al., 2019 [127] |
| MYD88 gene | ddPCR | PCNSL, n = 42 Diagnosis |
| Concordance | Yamagishi et al., 2021 [128] |
| RHOAG17V and IDH2R172 mutations | AS-PCR | PCNSL, n = 20 Diagnosis |
| Concordance Response assessment | Hayashida et al., 2020 [129] |
| TCR rearrangements | dPCR | PTCLs, n = 34: ALCL, n = 10 PTCL-NOS, n = 10 Other, n = 14 Diagnosis |
| Response assessment | Miljkovic et al., 2021 [130] |
| XPO1 gene | dPCR | cHL, n = 94 Diagnosis and post-treatment |
| Concordance Response assessment Prognosis MRD | Camus et al., 2016 [135] |
| STAT6 mutations | NGSCAPP-seq | cHl, n = 112 Newly diagnosed, n = 80 Refractory, n = 32 |
| Response assessment Prognosis | Spina et al., 2018 [134] |
| NFKBIE, TNFAIP3, STAT6, PTPN1, B2M, XPO1, ITPKB, GNA13, and SOCS1 genes | NGS | HL, n = 60 Diagnosis and post-treatment |
| Concordance Prognosis | Camus et al., 2021 [133] |
4.3. Multiple Myeloma
| Target | Methods | Cohort Size/Disease Stage | Evidence: Key Points | Application | Ref. |
|---|---|---|---|---|---|
| IgH gene | ASO-PCR | n = 30 Diagnosis and post-treatment |
| Concordance | Sata et al., 2015 [156] |
| V(D)J rearrangement | NGS | n = 27 Diagnosis and post-treatment |
| Concordance Prognosis | Oberle et al., 2017 [157] |
| KRAS, NRAS, BRAF, and TP53 mutations | ddPCR | n = 60 New diagnosis, n = 15 Relapse/refractory, n = 33 Diagnosis and post-treatment Normal volunteers, n = 12 |
| Concordance Response assessment | Mithraprabhu et al., 2017 [158] |
| NRAS, KRAS, and BRAF mutations | ddPCR | n = 18 Diagnosis and post-treatment |
| Concordance Response assessment | Rustad et al., 2017 [159] |
| KRAS, NRAS, BRAF, EGFR, and PIK3CA mutations | ddPCR | n = 53 New diagnosis, n = 11 Relapsed, n = 42 |
| Concordance Prognosis | Kis et al., 2017 [162] |
| Somatic mutations | CAPP-seq | n = 28 New diagnosis, n = 25 Relapsed/refractory, n = 3 Diagnosis |
| Concordance | Gerber et al., 2018 [160] |
| IgH gene rearrangements | ddPCR | n = 25 At first relapse |
| Concordance Prognosis | Biancon et al., 2018 [161] |
| IgH gene rearrangements | NGS | n = 37 Post-treatment |
| MRD | Mazzotti et al., 2018 [168] |
| Somatic mutations | WES | n = 163 cfDNA, n = 107 CTCs, n = 56 Diagnosis |
| Concordance | Manier et al., 2018 [170] |
| Somatic mutations | WES | n = 105 MM patients, n = 93 Healthy patients, n = 12 Diagnosis |
| Concordance | Guo et al., 2018 [171] |
| Somatic mutations | Ultra-deep NGS | n = 65 MGUS, n = 15 SMM, n = 33 MM, n = 17 Diagnosis |
| Concordance Prognosis | Manzoni et al., 2020 [166] |
| Somatic mutations | NGS | n =18 EMM patients, n = 8 MM without EM spread, n = 10 Diagnosis |
| Concordance | Long et al., 2020 [169] |
| Somatic mutations | Targeted NGS | n = 77 Newly diagnosed, n = 52 Relapsed, n = 2 Previously treated, n = 23 |
| Concordance Prognosis | Deshpande et al., 2021 [165] |
5. Current Situation and Preanalytical Recommendations
5.1. Current Situation
5.2. Preanalytical Recommendations
6. Discussion
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poulet, G.; Massias, J.; Taly, V. Liquid Biopsy: General Concepts. Acta Cytol. 2019, 63, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C.; Schwarzenbach, H.; Pantel, K. Circulating Tumor Cells and Circulating Tumor DNA. Annu. Rev. Med. 2012, 63, 199–215. [Google Scholar] [CrossRef]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating Liquid Biopsies into the Management of Cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bode, A.M.; Dong, Z. Circulating Tumor Cells: Moving Biological Insights into Detection. Theranostics 2017, 7, 2606–2619. [Google Scholar] [CrossRef]
- Cirillo, M.; Craig, A.F.M.; Borchmann, S.; Kurtz, D.M. Liquid Biopsy in Lymphoma: Molecular Methods and Clinical Applications. Cancer Treat. Rev. 2020, 91, 102106. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Sharifi, M. Exosomal MiRNAs as Novel Cancer Biomarkers: Challenges and Opportunities. J. Cell. Physiol. 2018, 233, 6370–6380. [Google Scholar] [CrossRef]
- Leslie, M. Cell Biology. Beyond Clotting: The Powers of Platelets. Science 2010, 328, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.J.A.; Balaj, L.; Hulleman, E.; van Rijn, S.; Pegtel, D.M.; Walraven, M.; Widmark, A.; Gerritsen, W.R.; Verheul, H.M.; Vandertop, W.P.; et al. Blood Platelets Contain Tumor-Derived RNA Biomarkers. Blood 2011, 118, 3680–3683. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef] [Green Version]
- Van Eijndhoven, M.A.; Zijlstra, J.M.; Groenewegen, N.J.; Drees, E.E.; van Niele, S.; Baglio, S.R.; Koppers-Lalic, D.; van der Voorn, H.; Libregts, S.F.; Wauben, M.H.; et al. Plasma Vesicle MiRNAs for Therapy Response Monitoring in Hodgkin Lymphoma Patients. JCI Insight 2016, 1, e89631. [Google Scholar] [CrossRef]
- Mandel, P.; Metais, P. Nuclear Acids In Human Blood Plasma. C. R. Seances Soc. Biol. Fil. 1948, 142, 241–243. [Google Scholar] [PubMed]
- Leon, S.A.; Shapiro, B.; Sklaroff, D.M.; Yaros, M.J. Free DNA in the Serum of Cancer Patients and the Effect of Therapy. Cancer Res. 1977, 37, 646–650. [Google Scholar] [PubMed]
- Stroun, M.; Anker, P.; Maurice, P.; Lyautey, J.; Lederrey, C.; Beljanski, M. Neoplastic Characteristics of the DNA Found in the Plasma of Cancer Patients. Oncology 1989, 46, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Khier, S.; Lohan, L. Kinetics of Circulating Cell-Free DNA for Biomedical Applications: Critical Appraisal of the Literature. Future Sci. OA 2018, 4, FSO295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stroun, M.; Lyautey, J.; Lederrey, C.; Olson-Sand, A.; Anker, P. About the Possible Origin and Mechanism of Circulating DNA. Clin. Chim. Acta 2001, 313, 139–142. [Google Scholar] [CrossRef]
- Van der Vaart, M.; Pretorius, P.J. The Origin of Circulating Free DNA. Clin. Chem. 2007, 53, 2215. [Google Scholar] [CrossRef] [Green Version]
- Van der Vaart, M.; Pretorius, P.J. Circulating DNA. Its Origin and Fluctuation. Ann. N. Y. Acad. Sci. 2008, 1137, 18–26. [Google Scholar] [CrossRef]
- Mouliere, F.; Chandrananda, D.; Piskorz, A.M.; Moore, E.K.; Morris, J.; Ahlborn, L.B.; Mair, R.; Goranova, T.; Marass, F.; Heider, K.; et al. Enhanced Detection of Circulating Tumor DNA by Fragment Size Analysis. Sci. Transl. Med. 2018, 10, eaat4921. [Google Scholar] [CrossRef] [Green Version]
- Jahr, S.; Hentze, H.; Englisch, S.; Hardt, D.; Fackelmayer, F.O.; Hesch, R.D.; Knippers, R. DNA Fragments in the Blood Plasma of Cancer Patients: Quantitations and Evidence for Their Origin from Apoptotic and Necrotic Cells. Cancer Res. 2001, 61, 1659–1665. [Google Scholar]
- Corcoran, R.B.; Chabner, B.A. Application of Cell-Free DNA Analysis to Cancer Treatment. N. Engl. J. Med. 2018, 379, 1754–1765. [Google Scholar] [CrossRef] [Green Version]
- Hohaus, S.; Giachelia, M.; Massini, G.; Mansueto, G.; Vannata, B.; Bozzoli, V.; Criscuolo, M.; D’Alò, F.; Martini, M.; Larocca, L.M.; et al. Cell-Free Circulating DNA in Hodgkin’s and Non-Hodgkin’s Lymphomas. Ann. Oncol. 2009, 20, 1408–1413. [Google Scholar] [CrossRef]
- Diaz, L.A.; Bardelli, A. Liquid Biopsies: Genotyping Circulating Tumor DNA. J. Clin. Oncol. 2014, 32, 579–586. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef] [Green Version]
- Hindson, C.M.; Chevillet, J.R.; Briggs, H.A.; Gallichotte, E.N.; Ruf, I.K.; Hindson, B.J.; Vessella, R.L.; Tewari, M. Absolute Quantification by Droplet Digital PCR versus Analog Real-Time PCR. Nat. Methods 2013, 10, 1003–1005. [Google Scholar] [CrossRef]
- Bratman, S.V.; Newman, A.M.; Alizadeh, A.A.; Diehn, M. Potential Clinical Utility of Ultrasensitive Circulating Tumor DNA Detection with CAPP-Seq. Expert Rev. Mol. Diagn. 2015, 15, 715–719. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, T.; Rachlis, E.; Bug, G.; Stelljes, M.; Klein, S.; Steckel, N.K.; Wolf, D.; Ringhoffer, M.; Czibere, A.; Nachtkamp, K.; et al. Treatment of Acute Myeloid Leukemia or Myelodysplastic Syndrome Relapse after Allogeneic Stem Cell Transplantation with Azacitidine and Donor Lymphocyte Infusions—A Retrospective Multicenter Analysis from the German Cooperative Transplant Study Group. Biol. Blood Marrow Transplant. 2015, 21, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Onecha, E.; Linares, M.; Rapado, I.; Ruiz-Heredia, Y.; Martinez-Sanchez, P.; Cedena, T.; Pratcorona, M.; Oteyza, J.P.; Herrera, P.; Barragan, E.; et al. A Novel Deep Targeted Sequencing Method for Minimal Residual Disease Monitoring in Acute Myeloid Leukemia. Haematologica 2019, 104, 288–296. [Google Scholar] [CrossRef]
- Onecha, E.; Rapado, I.; Luz Morales, M.; Carreño-Tarragona, G.; Martinez-Sanchez, P.; Gutierrez, X.; Sánchez Pina, J.M.; Linares, M.; Gallardo, M.; Martinez-López, J.; et al. Monitoring of Clonal Evolution of Acute Myeloid Leukemia Identifies the Leukemia Subtype, Clinical Outcome and Potential New Drug Targets for Post-Remission Strategies or Relapse. Haematologica 2021, 106, 2325–2333. [Google Scholar] [CrossRef]
- Shapiro, R.M.; Kim, D.D.H. Next-Generation Sequencing-Based Minimal Residual Disease Monitoring in Patients Receiving Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia or Myelodysplastic Syndrome. Curr. Opin. Hematol. 2018, 25, 425–432. [Google Scholar] [CrossRef]
- Platzbecker, U.; Kubasch, A.S.; Homer-Bouthiette, C.; Prebet, T. Current Challenges and Unmet Medical Needs in Myelodysplastic Syndromes. Leukemia 2021, 35, 2182–2198. [Google Scholar] [CrossRef]
- Honoré, N.; Galot, R.; van Marcke, C.; Limaye, N.; Machiels, J.-P. Liquid Biopsy to Detect Minimal Residual Disease: Methodology and Impact. Cancers 2021, 13, 5364. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Woo, H.I.; Kim, J.-W.; Md, Y.K.; Lee, K.-A. Clinical Practice Guidelines for Pre-Analytical Procedures of Plasma Epidermal Growth Factor Receptor Variant Testing. Ann. Lab. Med. 2022, 42, 141–149. [Google Scholar] [CrossRef]
- Heuser, M.; Freeman, S.D.; Ossenkoppele, G.J.; Buccisano, F.; Hourigan, C.S.; Ngai, L.L.; Tettero, J.M.; Bachas, C.; Baer, C.; Béné, M.-C.; et al. 2021 Update on MRD in Acute Myeloid Leukemia: A Consensus Document from the European LeukemiaNet MRD Working Party. Blood 2021, 138, 2753–2767. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Acute Myeloid Leukemia (Version 1.2022); National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2022. [Google Scholar]
- National Comprehensive Cancer Network. Myelodysplastic Syndromes (Version 3.2022); National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2022. [Google Scholar]
- Barosi, G.; Mesa, R.; Finazzi, G.; Harrison, C.; Kiladjian, J.-J.; Lengfelder, E.; McMullin, M.F.; Passamonti, F.; Vannucchi, A.M.; Besses, C.; et al. Revised Response Criteria for Polycythemia Vera and Essential Thrombocythemia: An ELN and IWG-MRT Consensus Project. Blood 2013, 121, 4778–4781. [Google Scholar] [CrossRef]
- Tefferi, A.; Cervantes, F.; Mesa, R.; Passamonti, F.; Verstovsek, S.; Vannucchi, A.M.; Gotlib, J.; Dupriez, B.; Pardanani, A.; Harrison, C.; et al. Revised Response Criteria for Myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) Consensus Report. Blood 2013, 122, 1395–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Comprehensive Cancer Network. Myeloproliferative Neoplasms (Version 2.2021); National Comprehensive Cancer Network: Plymouth Meeting, PA, USA., 2021. [Google Scholar]
- National Comprehensive Cancer Network. Chronic Myeloid Leukemia (Version 2.2022); National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2022. [Google Scholar]
- National Comprehensive Cancer Network. Acute Lymphoblastic Leukemia (Version 4.2021); National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2021. [Google Scholar]
- National Comprehensive Cancer Network. B-Cell Lymphomas (Version 5.2021); National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2021. [Google Scholar]
- National Comprehensive Cancer Network. Hodgkin Lymphoma (Version 1.2022); National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2022. [Google Scholar]
- National Comprehensive Cancer Network. Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (Version 1.2022); National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2022. [Google Scholar]
- National Comprehensive Cancer Network. Multiple Myeloma (Version 4.2022); National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2022. [Google Scholar]
- Rowe, J.M. Will New Agents Impact Survival in AML? Best Pract. Res. Clin. Haematol. 2019, 32, 101094. [Google Scholar] [CrossRef] [PubMed]
- Pelcovits, A.; Niroula, R. Acute Myeloid Leukemia: A Review. Rhode Isl. Med. J. 2020, 103, 38–40. [Google Scholar]
- De Kouchkovsky, I.; Abdul-Hay, M. Acute Myeloid Leukemia: A Comprehensive Review and 2016 Update. Blood Cancer J. 2016, 6, e441. [Google Scholar] [CrossRef]
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzmaurice, C.; Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 2006 to 2016: A Systematic Analysis for the Global Burden of Disease Study. J. Clin. Oncol. 2018, 36, 1568. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, D.; Weinberg, O.K. How I Investigate Acute Myeloid Leukemia. Int. J. Lab. Hematol. 2020, 42, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Pettersson, L.; Levéen, P.; Axler, O.; Dvorakova, D.; Juliusson, G.; Ehinger, M. Improved Minimal Residual Disease Detection by Targeted Quantitative Polymerase Chain Reaction in Nucleophosmin 1 Type a Mutated Acute Myeloid Leukemia: MFC vs. RQ-PCR in MRD Detection in AML. Genes Chromosomes Cancer 2016, 55, 750–766. [Google Scholar] [CrossRef]
- Handschuh, L.; Kaźmierczak, M.; Milewski, M.; Gïralski, M.; Łuczak, M.; Wojtaszewska, M.; Uszczyńska-Ratajczak, B.; Lewandowski, K.; Komarnicki, M.; Figlerowicz, M. Gene Expression Profiling of Acute Myeloid Leukemia Samples from Adult Patients with AML-M1 and -M2 through Boutique Microarrays, Real-Time PCR and Droplet Digital PCR. Int. J. Oncol. 2017, 52, 656–678. [Google Scholar] [CrossRef]
- Grassi, S.; Guerrini, F.; Ciabatti, E.; Puccetti, R.; Salehzadeh, S.; Metelli, M.R.; Di Vita, A.; Domenichini, C.; Caracciolo, F.; Orciuolo, E.; et al. Digital Droplet PCR Is a Specific and Sensitive Tool for Detecting IDH2 Mutations in Acute Myeloid LeuKemia Patients. Cancers 2020, 12, 1738. [Google Scholar] [CrossRef]
- Maurillo, L.; Buccisano, F.; Spagnoli, A.; Del Poeta, G.; Panetta, P.; Neri, B.; Del Principe, M.I.; Mazzone, C.; Consalvo, M.I.; Tamburini, A.; et al. Monitoring of Minimal Residual Disease in Adult Acute Myeloid Leukemia Using Peripheral Blood as an Alternative Source to Bone Marrow. Haematologica 2007, 92, 605–611. [Google Scholar] [CrossRef] [Green Version]
- Buccisano, F.; Maurillo, L.; Gattei, V.; Del Poeta, G.; Del Principe, M.I.; Cox, M.C.; Panetta, P.; Consalvo, M.I.; Mazzone, C.; Neri, B.; et al. The Kinetics of Reduction of Minimal Residual Disease Impacts on Duration of Response and Survival of Patients with Acute Myeloid Leukemia. Leukemia 2006, 20, 1783–1789. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.-L.; Pearce, D.; Smith, M.L.; Jenner, M.; Andrew Lister, T.; Bonnet, D.; Goff, L.; Fitzgibbon, J. Development of a Quantitative Real-Time Polymerase Chain Reaction Method for Monitoring CEBPA Mutations in Normal Karyotype Acute Myeloid Leukaemia. Br. J. Haematol. 2006, 133, 103–105. [Google Scholar] [CrossRef]
- Kern, W.; Bacher, U.; Haferlach, C.; Schnittger, S.; Haferlach, T. The Role of Multiparameter Flow Cytometry for Disease Monitoring in AML. Best Pract. Res. Clin. Haematol. 2010, 23, 379–390. [Google Scholar] [CrossRef]
- Cruz, N.M.; Mencia-Trinchant, N.; Hassane, D.C.; Guzman, M.L. Minimal Residual Disease in Acute Myelogenous Leukemia. Int. J. Lab. Hematol. 2017, 39, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Zeijlemaker, W.; Kelder, A.; Oussoren-Brockhoff, Y.J.M.; Scholten, W.J.; Snel, A.N.; Veldhuizen, D.; Cloos, J.; Ossenkoppele, G.J.; Schuurhuis, G.J. Peripheral Blood Minimal Residual Disease May Replace Bone Marrow Minimal Residual Disease as an Immunophenotypic Biomarker for Impending Relapse in Acute Myeloid Leukemia. Leukemia 2016, 30, 708–715. [Google Scholar] [CrossRef]
- Thol, F.; Kölking, B.; Damm, F.; Reinhardt, K.; Klusmann, J.-H.; Reinhardt, D.; von Neuhoff, N.; Brugman, M.H.; Schlegelberger, B.; Suerbaum, S.; et al. Next-Generation Sequencing for Minimal Residual Disease Monitoring in Acute Myeloid Leukemia Patients with FLT3-ITD or NPM1 Mutations. Genes Chromosomes Cancer 2012, 51, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Vonk, C.M.; Al Hinai, A.S.A.; Hanekamp, D.; Valk, P.J.M. Molecular Minimal Residual Disease Detection in Acute Myeloid Leukemia. Cancers 2021, 13, 5431. [Google Scholar] [CrossRef] [PubMed]
- Grimwade, D.; Freeman, S.D. Defining Minimal Residual Disease in Acute Myeloid Leukemia: Which Platforms Are Ready for “Prime Time”? Hematol. Am. Soc. Hematol. Educ. Program 2014, 2014, 222–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shumilov, E.; Flach, J.; Kohlmann, A.; Banz, Y.; Bonadies, N.; Fiedler, M.; Pabst, T.; Bacher, U. Current Status and Trends in the Diagnostics of AML and MDS. Blood Rev. 2018, 32, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Haferlach, T. Advancing Leukemia Diagnostics: Role of Next Generation Sequencing (NGS) in Acute Myeloid Leukemia. Hematol. Rep. 2020, 12, 8957. [Google Scholar] [CrossRef]
- Flach, J.; Shumilov, E.; Joncourt, R.; Porret, N.; Novak, U.; Pabst, T.; Bacher, U. Current Concepts and Future Directions for Hemato-Oncologic Diagnostics. Crit. Rev. Oncol. Hematol. 2020, 151, 102977. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, H. Next-Generation Sequencing in Liquid Biopsy: Cancer Screening and Early Detection. Hum. Genom. 2019, 13, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Short, N.J.; Patel, K.P.; Albitar, M.; Franquiz, M.; Luthra, R.; Kanagal-Shamanna, R.; Wang, F.; Assi, R.; Montalban-Bravo, G.; Matthews, J.; et al. Targeted Next-Generation Sequencing of Circulating Cell-Free DNA vs Bone Marrow in Patients with Acute Myeloid Leukemia. Blood Adv. 2020, 4, 1670–1677. [Google Scholar] [CrossRef]
- Nakamura, S.; Yokoyama, K.; Shimizu, E.; Yusa, N.; Kondoh, K.; Ogawa, M.; Takei, T.; Kobayashi, A.; Ito, M.; Isobe, M.; et al. Prognostic Impact of Circulating Tumor DNA Status Post–Allogeneic Hematopoietic Stem Cell Transplantation in AML and MDS. Blood 2019, 133, 2682–2695. [Google Scholar] [CrossRef]
- Rausch, C.; Rothenberg-Thurley, M.; Buerger, S.A.; Tschuri, S.; Dufour, A.; Neusser, M.; Schneider, S.; Spiekermann, K.; Metzeler, K.H.; Ziemann, F. Double Drop-Off Droplet Digital PCR. J. Mol. Diagn. 2021, 23, 975–985. [Google Scholar] [CrossRef]
- Lim, J.K.; Kuss, B.; Talaulikar, D. Role of Cell-Free DNA in Haematological Malignancies. Pathology 2021, 53, 416–426. [Google Scholar] [CrossRef]
- Steensma, D.P. Clinical Implications of Clonal Hematopoiesis. Mayo Clin. Proc. 2018, 93, 1122–1130. [Google Scholar] [CrossRef] [Green Version]
- Hasserjian, R.P.; Steensma, D.P.; Graubert, T.A.; Ebert, B.L. Clonal Hematopoiesis and Measurable Residual Disease Assessment in Acute Myeloid Leukemia. Blood 2020, 135, 1729–1738. [Google Scholar] [CrossRef]
- Yeh, P.; Dickinson, M.; Ftouni, S.; Hunter, T.; Sinha, D.; Wong, S.Q.; Agarwal, R.; Vedururu, R.; Doig, K.; Fong, C.Y.; et al. Molecular Disease Monitoring Using Circulating Tumor DNA in Myelodysplastic Syndromes. Blood 2017, 129, 1685–1690. [Google Scholar] [CrossRef]
- Gutierrez-Rodrigues, F.; Beerman, I.; Groarke, E.M.; Patel, B.A.; Spitofsky, N.; Dillon, L.W.; Raffo, D.Q.; Hourigan, C.S.; Kajigaya, S.; Ferrucci, L.; et al. Utility of Plasma Cell-Free DNA for de Novo Detection and Quantification of Clonal Hematopoiesis. Haematologica 2021. early view. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 Revision to the World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Barbui, T.; Thiele, J.; Gisslinger, H.; Kvasnicka, H.M.; Vannucchi, A.M.; Guglielmelli, P.; Orazi, A.; Tefferi, A. The 2016 WHO Classification and Diagnostic Criteria for Myeloproliferative Neoplasms: Document Summary and in-Depth Discussion. Blood Cancer J. 2018, 8, 15. [Google Scholar] [CrossRef]
- Tefferi, A.; Barbui, T. Polycythemia Vera and Essential Thrombocythemia: 2021 Update on Diagnosis, Risk-stratification and Management. Am. J. Hematol. 2020, 95, 1599–1613. [Google Scholar] [CrossRef]
- Garcia-Gisbert, N.; Fernández-Ibarrondo, L.; Fernández-Rodríguez, C.; Gibert, J.; Andrade-Campos, M.; Arenillas, L.; Camacho, L.; Angona, A.; Longarón, R.; Salar, A.; et al. Circulating Cell-Free DNA Improves the Molecular Characterisation of Ph-Negative Myeloproliferative Neoplasms. Br. J. Haematol. 2021, 192, 300–309. [Google Scholar] [CrossRef]
- Redaelli, A.; Laskin, B.L.; Stephens, J.M.; Botteman, M.F.; Pashos, C.L. A Systematic Literature Review of the Clinical and Epidemiological Burden of Acute Lymphoblastic Leukaemia (ALL). Eur. J. Cancer Care 2005, 14, 53–62. [Google Scholar] [CrossRef]
- Nunes, V.; Cazzaniga, G.; Biondi, A. An Update on PCR Use for Minimal Residual Disease Monitoring in Acute Lymphoblastic Leukemia. Expert Rev. Mol. Diagn. 2017, 17, 953–963. [Google Scholar] [CrossRef]
- Theunissen, P.; Mejstrikova, E.; Sedek, L.; van der Sluijs-Gelling, A.J.; Gaipa, G.; Bartels, M.; Sobral da Costa, E.; Kotrová, M.; Novakova, M.; Sonneveld, E.; et al. Standardized Flow Cytometry for Highly Sensitive MRD Measurements in B-Cell Acute Lymphoblastic Leukemia. Blood 2017, 129, 347–357. [Google Scholar] [CrossRef]
- Kruse, A.; Abdel-Azim, N.; Kim, H.N.; Ruan, Y.; Phan, V.; Ogana, H.; Wang, W.; Lee, R.; Gang, E.J.; Khazal, S.; et al. Minimal Residual Disease Detection in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2020, 21, 1054. [Google Scholar] [CrossRef] [Green Version]
- Kotrova, M.; Muzikova, K.; Mejstrikova, E.; Novakova, M.; Bakardjieva-Mihaylova, V.; Fiser, K.; Stuchly, J.; Giraud, M.; Salson, M.; Pott, C.; et al. The Predictive Strength of Next-Generation Sequencing MRD Detection for Relapse Compared with Current Methods in Childhood ALL. Blood 2015, 126, 1045–1047. [Google Scholar] [CrossRef]
- Muffly, L.; Sundaram, V.; Chen, C.; Yurkiewicz, I.; Kuo, E.; Burnash, S.; Spiegel, J.Y.; Arai, S.; Frank, M.J.; Johnston, L.J.; et al. Concordance of Peripheral Blood and Bone Marrow Measurable Residual Disease in Adult Acute Lymphoblastic Leukemia. Blood Adv. 2021, 5, 3147–3151. [Google Scholar] [CrossRef]
- Van der Velden, V.H.J.; Jacobs, D.C.H.; Wijkhuijs, A.J.M.; Comans-Bitter, W.M.; Willemse, M.J.; Hählen, K.; Kamps, W.A.; van Wering, E.R.; van Dongen, J.J.M. Minimal Residual Disease Levels in Bone Marrow and Peripheral Blood Are Comparable in Children with T Cell Acute Lymphoblastic Leukemia (ALL), but Not in Precursor-B-ALL. Leukemia 2002, 16, 1432–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, A.K.; Stanulla, M.; Cario, G.; Flohr, T.; Sutton, R.; Möricke, A.; Anker, P.; Stroun, M.; Welte, K.; Bartram, C.R.; et al. Quantification of Free Total Plasma DNA and Minimal Residual Disease Detection in the Plasma of Children with Acute Lymphoblastic Leukemia. Ann. Hematol. 2009, 88, 897–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.H.; Lau, K.M.; Li, C.K.; Chan, N.P.H.; Ip, R.K.L.; Cheng, C.K.; Lee, V.; Shing, M.M.K.; Leung, A.W.K.; Ha, S.Y.; et al. Minimal Residual Disease-Based Risk Stratification in Chinese Childhood Acute Lymphoblastic Leukemia by Flow Cytometry and Plasma DNA Quantitative Polymerase Chain Reaction. PLoS ONE 2013, 8, e69467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yegin, Z.A.; Can, F.; Gökçen, S.; Sadioğlu, R.E.; Özkurt, Z.N.; İlhan, Ç.; Yağcı, M. The Impact of Pre-Transplant Cell-Free DNA Levels on Leukemia Relapse and Transplant-Related Complications in Allogeneic Hematopoietic Stem Cell Transplant Recipients. Balk. Med. J. 2020, 37, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Camus, V.; Jardin, F. Cell-Free DNA for the Management of Classical Hodgkin Lymphoma. Pharm. Basel Switz. 2021, 14, 207. [Google Scholar] [CrossRef]
- Schürch, C.M.; Federmann, B.; Quintanilla-Martinez, L.; Fend, F. Tumor Heterogeneity in Lymphomas: A Different Breed. Pathobiology 2018, 85, 130–145. [Google Scholar] [CrossRef]
- Melani, C.; Wilson, W.H.; Roschewski, M. Monitoring Clinical Outcomes in Aggressive B-Cell Lymphoma: From Imaging Studies to Circulating Tumor DNA. Best Pract. Res. Clin. Haematol. 2018, 31, 285–292. [Google Scholar] [CrossRef]
- Camus, V.; Jardin, F.; Tilly, H. The Value of Liquid Biopsy in Diagnosis and Monitoring of Diffuse Large B-Cell Lymphoma: Recent Developments and Future Potential. Expert Rev. Mol. Diagn. 2017, 17, 557–566. [Google Scholar] [CrossRef]
- Li, M.; Xu, C. Circulating Cell-Free DNA Utility for the Surveillance of Patients with Treated Diffuse Large B-Cell Lymphoma. Clin. Oncol. R. Coll. Radiol. G. B. 2017, 29, 637–638. [Google Scholar] [CrossRef]
- Eskandari, M.; Manoochehrabadi, S.; Pashaiefar, H.; Zaimy, M.A.; Ahmadvand, M. Clinical Significance of Cell-Free DNA as a Prognostic Biomarker in Patients with Diffuse Large B-Cell Lymphoma. Blood Res. 2019, 54, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Hur, J.Y.; Kim, Y.J.; Yoon, S.E.; Son, D.-S.; Park, W.-Y.; Kim, S.J.; Park, D.; Kim, W.S. Plasma Cell-Free DNA Is a Prognostic Biomarker for Survival in Patients with Aggressive Non-Hodgkin Lymphomas. Ann. Hematol. 2020, 99, 1293–1302. [Google Scholar] [CrossRef]
- Lv, L.; Liu, Y. Clinical Application of Liquid Biopsy in Non-Hodgkin Lymphoma. Front. Oncol. 2021, 11, 658234. [Google Scholar] [CrossRef]
- Armand, P.; Oki, Y.; Neuberg, D.S.; Faham, M.; Cummings, C.; Klinger, M.; Weng, L.; Bhattar, S.; Lacasce, A.S.; Jacobsen, E.D.; et al. Detection of Circulating Tumour DNA in Patients with Aggressive B-Cell Non-Hodgkin Lymphoma. Br. J. Haematol. 2013, 163, 123–126. [Google Scholar] [CrossRef]
- Kurtz, D.M.; Green, M.R.; Bratman, S.V.; Scherer, F.; Liu, C.L.; Kunder, C.A.; Takahashi, K.; Glover, C.; Keane, C.; Kihira, S.; et al. Noninvasive Monitoring of Diffuse Large B-Cell Lymphoma by Immunoglobulin High-Throughput Sequencing. Blood 2015, 125, 3679–3687. [Google Scholar] [CrossRef] [Green Version]
- Roschewski, M.; Dunleavy, K.; Pittaluga, S.; Moorhead, M.; Pepin, F.; Kong, K.; Shovlin, M.; Jaffe, E.S.; Staudt, L.M.; Lai, C.; et al. Circulating Tumour DNA and CT Monitoring in Patients with Untreated Diffuse Large B-Cell Lymphoma: A Correlative Biomarker Study. Lancet Oncol. 2015, 16, 541–549. [Google Scholar] [CrossRef] [Green Version]
- Herrera, A.F.; Kim, H.T.; Kong, K.A.; Faham, M.; Sun, H.; Sohani, A.R.; Alyea, E.P.; Carlton, V.E.; Chen, Y.-B.; Cutler, C.S.; et al. Next-Generation Sequencing-Based Detection of Circulating Tumour DNA After Allogeneic Stem Cell Transplantation for Lymphoma. Br. J. Haematol. 2016, 175, 841–850. [Google Scholar] [CrossRef]
- Camus, V.; Jardin, F. Cell-Free DNA and the Monitoring of Lymphoma Treatment. Pharmacogenomics 2019, 20, 1271–1282. [Google Scholar] [CrossRef]
- Li, M.; Jia, Y.; Xu, J.; Cheng, X.; Xu, C. Assessment of the Circulating Cell-Free DNA Marker Association with Diagnosis and Prognostic Prediction in Patients with Lymphoma: A Single-Center Experience. Ann. Hematol. 2017, 96, 1343–1351. [Google Scholar] [CrossRef] [Green Version]
- Rossi, D.; Diop, F.; Spaccarotella, E.; Monti, S.; Zanni, M.; Rasi, S.; Deambrogi, C.; Spina, V.; Bruscaggin, A.; Favini, C.; et al. Diffuse Large B-Cell Lymphoma Genotyping on the Liquid Biopsy. Blood 2017, 129, 1947–1957. [Google Scholar] [CrossRef]
- Kurtz, D.M.; Scherer, F.; Jin, M.C.; Soo, J.; Craig, A.F.M.; Esfahani, M.S.; Chabon, J.J.; Stehr, H.; Liu, C.L.; Tibshirani, R.; et al. Circulating Tumor DNA Measurements As Early Outcome Predictors in Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2018, 36, 2845–2853. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Delgado, A.; Nadeu, F.; Enjuanes, A.; Casanueva-Eliceiry, S.; Mozas, P.; Magnano, L.; Castrejón de Anta, N.; Rovira, J.; Dlouhy, I.; Martín, S.; et al. Mutational Landscape and Tumor Burden Assessed by Cell-Free DNA in Diffuse Large B-Cell Lymphoma in a Population-Based Study. Clin. Cancer Res. 2021, 27, 513–521. [Google Scholar] [CrossRef]
- Kurtz, D.M.; Soo, J.; Co Ting Keh, L.; Alig, S.; Chabon, J.J.; Sworder, B.J.; Schultz, A.; Jin, M.C.; Scherer, F.; Garofalo, A.; et al. Enhanced Detection of Minimal Residual Disease by Targeted Sequencing of Phased Variants in Circulating Tumor DNA. Nat. Biotechnol. 2021, 39, 1537–1547. [Google Scholar] [CrossRef]
- Scherer, F.; Kurtz, D.M.; Newman, A.M.; Stehr, H.; Craig, A.F.M.; Esfahani, M.S.; Lovejoy, A.F.; Chabon, J.J.; Klass, D.M.; Liu, C.L.; et al. Distinct Biological Subtypes and Patterns of Genome Evolution in Lymphoma Revealed by Circulating Tumor DNA. Sci. Transl. Med. 2016, 8, 364ra155. [Google Scholar] [CrossRef] [Green Version]
- Arzuaga-Mendez, J.; Prieto-Fernández, E.; Lopez-Lopez, E.; Martin-Guerrero, I.; García-Ruiz, J.C.; García-Orad, A. Cell-Free DNA as a Biomarker in Diffuse Large B-Cell Lymphoma: A Systematic Review. Crit. Rev. Oncol. Hematol. 2019, 139, 7–15. [Google Scholar] [CrossRef]
- Chen, F.; Pang, D.; Guo, H.; Jiang, X.; Liu, S.; Huang, L.; Wei, X.; Liang, Z.; Wang, X.; Li, W. Clinicopathological Characteristics and Mutational Profiling of Adult T-Cell Lymphoblastic Lymphoma in a Chinese Population. Cancer Manag. Res. 2020, 12, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-H.; Kim, Y.J.; Lee, D.; Cho, D.; Ko, Y.H.; Cho, J.; Park, W.-Y.; Park, D.; Kim, S.J.; Kim, W.S. Analysis of Circulating Tumor DNA by Targeted Ultra-Deep Sequencing across Various Non-Hodgkin Lymphoma Subtypes. Leuk. Lymphoma 2019, 60, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Suehara, Y.; Sakata-Yanagimoto, M.; Hattori, K.; Kusakabe, M.; Nanmoku, T.; Sato, T.; Noguchi, M.; Chiba, S. Mutations Found in Cell-free DNAs of Patients with Malignant Lymphoma at Remission Can Derive from Clonal Hematopoiesis. Cancer Sci. 2019, 110, 3375–3381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, N.M.; Dahiya, S.; Le, R.; Abramian, A.M.; Kong, K.A.; Muffly, L.S.; Miklos, D.B. Circulating Tumor DNA Assessment in Patients with Diffuse Large B-Cell Lymphoma Following CAR T-Cell Therapy. Leuk. Lymphoma 2019, 60, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, S.; Genuardi, E.; Mazziotta, F.; Iovino, L.; Morabito, F.; Grassi, S.; Ciabatti, E.; Guerrini, F.; Petrini, M. The Minimal Residual Disease in Non-Hodgkin’s Lymphomas: From the Laboratory to the Clinical Practice. Front. Oncol. 2019, 9, 528. [Google Scholar] [CrossRef]
- Cowan, A.J.; Stevenson, P.A.; Cassaday, R.D.; Graf, S.A.; Fromm, J.R.; Wu, D.; Holmberg, L.A.; Till, B.G.; Chauncey, T.R.; Smith, S.D.; et al. Pretransplantation Minimal Residual Disease Predicts Survival in Patients with Mantle Cell Lymphoma Undergoing Autologous Stem Cell Transplantation in Complete Remission. Biol. Blood Marrow Transplant. 2016, 22, 380–385. [Google Scholar] [CrossRef]
- Kolstad, A.; Pedersen, L.B.; Eskelund, C.W.; Husby, S.; Grønbæk, K.; Jerkeman, M.; Laurell, A.; Räty, R.; Elonen, E.; Andersen, N.S.; et al. Molecular Monitoring after Autologous Stem Cell Transplantation and Preemptive Rituximab Treatment of Molecular Relapse; Results from the Nordic Mantle Cell Lymphoma Studies (MCL2 and MCL3) with Median Follow-Up of 8.5 Years. Biol. Blood Marrow Transplant. 2017, 23, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Jung, D.; Jain, P.; Yao, Y.; Wang, M. Advances in the Assessment of Minimal Residual Disease in Mantle Cell Lymphoma. J. Hematol. Oncol. 2020, 13, 127. [Google Scholar] [CrossRef]
- Lakhotia, R.; Melani, C.; Pittaluga, S.; Dunleavy, K.; Saba, N.S.; Lucas, A.N.; Jacob, A.; Yusko, E.; Steinberg, S.M.; Jaffe, E.S.; et al. Circulating Tumor DNA Dynamics during Therapy Predict Outcomes in Mantle Cell Lymphoma. Blood 2018, 132, 147. [Google Scholar] [CrossRef]
- Agarwal, R.; Chan, Y.-C.; Tam, C.S.; Hunter, T.; Vassiliadis, D.; Teh, C.E.; Thijssen, R.; Yeh, P.; Wong, S.Q.; Ftouni, S.; et al. Dynamic Molecular Monitoring Reveals That SWI–SNF Mutations Mediate Resistance to Ibrutinib plus Venetoclax in Mantle Cell Lymphoma. Nat. Med. 2019, 25, 119–129. [Google Scholar] [CrossRef]
- Bachy, E.; Houot, R.; Morschhauser, F.; Sonet, A.; Brice, P.; Belhadj, K.; Cartron, G.; Audhuy, B.; Fermé, C.; Feugier, P.; et al. Long-Term Follow up of the FL2000 Study Comparing CHVP-Interferon to CHVP-Interferon plus Rituximab in Follicular Lymphoma. Haematologica 2013, 98, 1107–1114. [Google Scholar] [CrossRef]
- Sarkozy, C.; Huet, S.; Carlton, V.E.H.; Fabiani, B.; Delmer, A.; Jardin, F.; Delfau-Larue, M.-H.; Hacini, M.; Ribrag, V.; Guidez, S.; et al. The Prognostic Value of Clonal Heterogeneity and Quantitative Assessment of Plasma Circulating Clonal IG-VDJ Sequences at Diagnosis in Patients with Follicular Lymphoma. Oncotarget 2017, 8, 8765–8774. [Google Scholar] [CrossRef] [Green Version]
- Delfau-Larue, M.-H.; van der Gucht, A.; Dupuis, J.; Jais, J.-P.; Nel, I.; Beldi-Ferchiou, A.; Hamdane, S.; Benmaad, I.; Laboure, G.; Verret, B.; et al. Total Metabolic Tumor Volume, Circulating Tumor Cells, Cell-Free DNA: Distinct Prognostic Value in Follicular Lymphoma. Blood Adv. 2018, 2, 807–816. [Google Scholar] [CrossRef]
- Jimenez-Ubieto, A.I.; Heredia, Y.; de la Rosa, J.M.; Rodriguez-Izquierdo, A.; Rufian, L.; Carrillo, J.; Sanchez, R.; Onecha, E.; Wang, C.; Sarandeses, P.; et al. Minimal Residual Disease Monitoring from Liquid Biopsy By Next Generation Sequencing in Follicular Lymphoma Patients. In Proceedings of the 62nd ASH Annual Meeting and Exposition, Virtual, 5–8 December 2020. 282. [Google Scholar]
- Zeremski, V.; Koehler, M.; Fischer, T.; Schalk, E. Characteristics and Outcome of Patients with Primary CNS Lymphoma in a “Real-Life” Setting Compared to a Clinical Trial. Ann. Hematol. 2016, 95, 793–799. [Google Scholar] [CrossRef]
- Hattori, K.; Sakata-Yanagimoto, M.; Suehara, Y.; Yokoyama, Y.; Kato, T.; Kurita, N.; Nishikii, H.; Obara, N.; Takano, S.; Ishikawa, E.; et al. Clinical Significance of Disease-Specific MYD88 Mutations in Circulating DNA in Primary Central Nervous System Lymphoma. Cancer Sci. 2018, 109, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.E.; Kim, Y.J.; Shim, J.H.; Park, D.; Cho, J.; Ko, Y.H.; Park, W.-Y.; Mun, Y.-C.; Lee, K.E.; Cho, D.; et al. Plasma Circulating Tumor DNA in Patients with Primary Central Nervous System Lymphoma. Cancer Res. Treat. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Hiemcke-Jiwa, L.S.; Leguit, R.J.; Snijders, T.J.; Bromberg, J.E.C.; Nierkens, S.; Jiwa, N.M.; Minnema, M.C.; Huibers, M.M.H. MYD88 p.(L265P) Detection on Cell-Free DNA in Liquid Biopsies of Patients with Primary Central Nervous System Lymphoma. Br. J. Haematol. 2019, 185, 974–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimelen, V.; Ahle, G.; Pencreach, E.; Zinniger, N.; Debliquis, A.; Zalmaï, L.; Harzallah, I.; Hurstel, R.; Alamome, I.; Lamy, F.; et al. Tumor Cell-Free DNA Detection in CSF for Primary CNS Lymphoma Diagnosis. Acta Neuropathol. Commun. 2019, 7, 43. [Google Scholar] [CrossRef]
- Yamagishi, Y.; Sasaki, N.; Nakano, Y.; Matushita, Y.; Omura, T.; Shimizu, S.; Saito, K.; Kobayashi, K.; Narita, Y.; Kondo, A.; et al. Liquid Biopsy of Cerebrospinal Fluid for MYD88 L265P Mutation Is Useful for Diagnosis of Central Nervous System Lymphoma. Cancer Sci. 2021, 112, 4702–4710. [Google Scholar] [CrossRef]
- Hayashida, M.; Maekawa, F.; Chagi, Y.; Iioka, F.; Kobashi, Y.; Watanabe, M.; Ohno, H. Combination of Multicolor Flow Cytometry for Circulating Lymphoma Cells and Tests for the RHOAG17V and IDH2R172 Hot-Spot Mutations in Plasma Cell-Free DNA as Liquid Biopsy for the Diagnosis of Angioimmunoblastic T-Cell Lymphoma. Leuk. Lymphoma 2020, 61, 2389–2398. [Google Scholar] [CrossRef]
- Miljkovic, M.D.; Melani, C.; Pittaluga, S.; Lakhotia, R.; Lucas, N.; Jacob, A.; Yusko, E.; Jaffe, E.S.; Wilson, W.H.; Roschewski, M. Next-Generation Sequencing-Based Monitoring of Circulating Tumor DNA Reveals Clonotypic Heterogeneity in Untreated PTCL. Blood Adv. 2021, 5, 4198–4210. [Google Scholar] [CrossRef]
- Diehl, V.; Thomas, R.K.; Re, D. Part II: Hodgkin’s Lymphoma--Diagnosis and Treatment. Lancet Oncol. 2004, 5, 19–26. [Google Scholar] [CrossRef]
- Bessi, L.; Viailly, P.-J.; Bohers, E.; Ruminy, P.; Maingonnat, C.; Bertrand, P.; Vasseur, N.; Beaussire, L.; Cornic, M.; Etancelin, P.; et al. Somatic Mutations of Cell-Free Circulating DNA Detected by Targeted next-Generation Sequencing and Digital Droplet PCR in Classical Hodgkin Lymphoma. Leuk. Lymphoma 2019, 60, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Camus, V.; Viennot, M.; Lequesne, J.; Viailly, P.-J.; Bohers, E.; Bessi, L.; Marcq, B.; Etancelin, P.; Dubois, S.; Picquenot, J.-M.; et al. Targeted Genotyping of Circulating Tumor DNA for Classical Hodgkin Lymphoma Monitoring: A Prospective Study. Haematologica 2021, 106, 154–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spina, V.; Bruscaggin, A.; Cuccaro, A.; Martini, M.; Di Trani, M.; Forestieri, G.; Manzoni, M.; Condoluci, A.; Arribas, A.; Terzi-Di-Bergamo, L.; et al. Circulating Tumor DNA Reveals Genetics, Clonal Evolution, and Residual Disease in Classical Hodgkin Lymphoma. Blood 2018, 131, 2413–2425. [Google Scholar] [CrossRef] [Green Version]
- Camus, V.; Stamatoullas, A.; Mareschal, S.; Viailly, P.-J.; Sarafan-Vasseur, N.; Bohers, E.; Dubois, S.; Picquenot, J.M.; Ruminy, P.; Maingonnat, C.; et al. Detection and Prognostic Value of Recurrent Exportin 1 Mutations in Tumor and Cell-Free Circulating DNA of Patients with Classical Hodgkin Lymphoma. Haematologica 2016, 101, 1094–1101. [Google Scholar] [CrossRef]
- Baumann, T.; Delgado, J.; Santacruz, R.; Martínez-Trillos, A.; Royo, C.; Navarro, A.; Pinyol, M.; Rozman, M.; Pereira, A.; Villamor, N.; et al. Chronic Lymphocytic Leukemia in the Elderly: Clinico-Biological Features, Outcomes, and Proposal of a Prognostic Model. Haematologica 2014, 99, 1599–1604. [Google Scholar] [CrossRef] [Green Version]
- Böttcher, S.; Ritgen, M.; Fischer, K.; Stilgenbauer, S.; Busch, R.M.; Fingerle-Rowson, G.; Fink, A.M.; Bühler, A.; Zenz, T.; Wenger, M.K.; et al. Minimal Residual Disease Quantification Is an Independent Predictor of Progression-Free and Overall Survival in Chronic Lymphocytic Leukemia: A Multivariate Analysis from the Randomized GCLLSG CLL8 Trial. J. Clin. Oncol. 2012, 30, 980–988. [Google Scholar] [CrossRef]
- Ghia, P. A Look into the Future: Can Minimal Residual Disease Guide Therapy and Predict Prognosis in Chronic Lymphocytic Leukemia? Hematol. Am. Soc. Hematol. Educ. Program 2012, 2012, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Strati, P.; Keating, M.J.; O’Brien, S.M.; Burger, J.; Ferrajoli, A.; Jain, N.; Tambaro, F.P.; Estrov, Z.; Jorgensen, J.; Challagundla, P.; et al. Eradication of Bone Marrow Minimal Residual Disease May Prompt Early Treatment Discontinuation in CLL. Blood 2014, 123, 3727–3732. [Google Scholar] [CrossRef] [Green Version]
- Del Giudice, I.; Raponi, S.; Della Starza, I.; De Propris, M.S.; Cavalli, M.; De Novi, L.A.; Cappelli, L.V.; Ilari, C.; Cafforio, L.; Guarini, A.; et al. Minimal Residual Disease in Chronic Lymphocytic Leukemia: A New Goal? Front. Oncol. 2019, 9, 689. [Google Scholar] [CrossRef] [Green Version]
- Landgren, O.; Kyle, R.A.; Pfeiffer, R.M.; Katzmann, J.A.; Caporaso, N.E.; Hayes, R.B.; Dispenzieri, A.; Kumar, S.; Clark, R.J.; Baris, D.; et al. Monoclonal Gammopathy of Undetermined Significance (MGUS) Consistently Precedes Multiple Myeloma: A Prospective Study. Blood 2009, 113, 5412–5417. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.K.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Pandey, S.; Kapoor, P.; Dingli, D.; Hayman, S.R.; Leung, N.; et al. Continued Improvement in Survival in Multiple Myeloma: Changes in Early Mortality and Outcomes in Older Patients. Leukemia 2014, 28, 1122–1128. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group Consensus Criteria for Response and Minimal Residual Disease Assessment in Multiple Myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Flores-Montero, J.; Sanoja-Flores, L.; Paiva, B.; Puig, N.; García-Sánchez, O.; Böttcher, S.; van der Velden, V.H.J.; Pérez-Morán, J.-J.; Vidriales, M.-B.; García-Sanz, R.; et al. Next Generation Flow for Highly Sensitive and Standardized Detection of Minimal Residual Disease in Multiple Myeloma. Leukemia 2017, 31, 2094–2103. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.C.; Auclair, D.; Kelloff, G.J.; Sigman, C.C.; Avet-Loiseau, H.; Farrell, A.T.; Gormley, N.J.; Kumar, S.K.; Landgren, O.; Munshi, N.C.; et al. The Role of Minimal Residual Disease Testing in Myeloma Treatment Selection and Drug Development: Current Value and Future Applications. Clin. Cancer Res. 2017, 23, 3980–3993. [Google Scholar] [CrossRef] [Green Version]
- Kubaczkova, V.; Vrabel, D.; Sedlarikova, L.; Besse, L.; Sevcikova, S. Cell-Free DNA—Minimally Invasive Marker of Hematological Malignancies. Eur. J. Haematol. 2017, 99, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.W.; Shah, N.; Ledergor, G.; Martin, T.; Wolf, J.; Shui, A.M.; Huang, C.-Y.; Martinez-Lopez, J. Early Dynamics and Depth of Response in Multiple Myeloma Patients Treated With BCMA CAR-T Cells. Front. Oncol. 2021, 11, 783703. [Google Scholar] [CrossRef]
- Bolli, N.; Avet-Loiseau, H.; Wedge, D.C.; Van Loo, P.; Alexandrov, L.B.; Martincorena, I.; Dawson, K.J.; Iorio, F.; Nik-Zainal, S.; Bignell, G.R.; et al. Heterogeneity of Genomic Evolution and Mutational Profiles in Multiple Myeloma. Nat. Commun. 2014, 5, 2997. [Google Scholar] [CrossRef] [Green Version]
- Walker, B.A.; Wardell, C.P.; Melchor, L.; Brioli, A.; Johnson, D.C.; Kaiser, M.F.; Mirabella, F.; Lopez-Corral, L.; Humphray, S.; Murray, L.; et al. Intraclonal Heterogeneity Is a Critical Early Event in the Development of Myeloma and Precedes the Development of Clinical Symptoms. Leukemia 2014, 28, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Ghobrial, I.M. Myeloma as a Model for the Process of Metastasis: Implications for Therapy. Blood 2012, 120, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Beasley, S.; Prigozhina, N.L.; Higgins, R.; Ikeda, S.; Lee, F.Y.; Marrinucci, D.; Jia, S. Detection and Characterization of Circulating Tumour Cells in Multiple Myeloma. J. Circ. Biomark. 2016, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Lohr, J.G.; Kim, S.; Gould, J.; Knoechel, B.; Drier, Y.; Cotton, M.J.; Gray, D.; Birrer, N.; Wong, B.; Ha, G.; et al. Genetic Interrogation of Circulating Multiple Myeloma Cells at Single-Cell Resolution. Sci. Transl. Med. 2016, 8, 363ra147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foulk, B.; Schaffer, M.; Gross, S.; Rao, C.; Smirnov, D.; Connelly, M.C.; Chaturvedi, S.; Reddy, M.; Brittingham, G.; Mata, M.; et al. Enumeration and Characterization of Circulating Multiple Myeloma Cells in Patients with Plasma Cell Disorders. Br. J. Haematol. 2018, 180, 71–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcés, J.-J.; Simicek, M.; Vicari, M.; Brozova, L.; Burgos, L.; Bezdekova, R.; Alignani, D.; Calasanz, M.-J.; Growkova, K.; Goicoechea, I.; et al. Transcriptional Profiling of Circulating Tumor Cells in Multiple Myeloma: A New Model to Understand Disease Dissemination. Leukemia 2020, 34, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Terpos, E.; Fotiou, D.; Kastritis, E.; Dimopoulos, M.A. Monitoring Plasma Cell Dyscrasias with Cell-Free DNA Analysis. Clin. Lymphoma Myeloma Leuk. 2020, 20, e905–e909. [Google Scholar] [CrossRef] [PubMed]
- Sata, H.; Shibayama, H.; Maeda, I.; Habuchi, Y.; Nakatani, E.; Fukushima, K.; Fujita, J.; Ezoe, S.; Tadokoro, S.; Maeda, T.; et al. Quantitative Polymerase Chain Reaction Analysis with Allele-Specific Oligonucleotide Primers for Individual IgH VDJ Regions to Evaluate Tumor Burden in Myeloma Patients. Exp. Hematol. 2015, 43, 374–381.e2. [Google Scholar] [CrossRef]
- Oberle, A.; Brandt, A.; Voigtlaender, M.; Thiele, B.; Radloff, J.; Schulenkorf, A.; Alawi, M.; Akyüz, N.; März, M.; Ford, C.T.; et al. Monitoring Multiple Myeloma by Next-Generation Sequencing of V(D)J Rearrangements from Circulating Myeloma Cells and Cell-Free Myeloma DNA. Haematologica 2017, 102, 1105–1111. [Google Scholar] [CrossRef]
- Mithraprabhu, S.; Khong, T.; Ramachandran, M.; Chow, A.; Klarica, D.; Mai, L.; Walsh, S.; Broemeling, D.; Marziali, A.; Wiggin, M.; et al. Circulating Tumour DNA Analysis Demonstrates Spatial Mutational Heterogeneity That Coincides with Disease Relapse in Myeloma. Leukemia 2017, 31, 1695–1705. [Google Scholar] [CrossRef]
- Rustad, E.H.; Coward, E.; Skytøen, E.R.; Misund, K.; Holien, T.; Standal, T.; Børset, M.; Beisvag, V.; Myklebost, O.; Meza-Zepeda, L.A.; et al. Monitoring Multiple Myeloma by Quantification of Recurrent Mutations in Serum. Haematologica 2017, 102, 1266–1272. [Google Scholar] [CrossRef] [Green Version]
- Gerber, B.; Manzoni, M.; Spina, V.; Bruscaggin, A.; Lionetti, M.; Fabris, S.; Barbieri, M.; Ciceri, G.; Pompa, A.; Forestieri, G.; et al. Circulating Tumor DNA as a Liquid Biopsy in Plasma Cell Dyscrasias. Haematologica 2018, 103, e245–e248. [Google Scholar] [CrossRef] [Green Version]
- Biancon, G.; Gimondi, S.; Vendramin, A.; Carniti, C.; Corradini, P. Noninvasive Molecular Monitoring in Multiple Myeloma Patients Using Cell-Free Tumor DNA. J. Mol. Diagn. 2018, 20, 859–870. [Google Scholar] [CrossRef] [Green Version]
- Kis, O.; Kaedbey, R.; Chow, S.; Danesh, A.; Dowar, M.; Li, T.; Li, Z.; Liu, J.; Mansour, M.; Masih-Khan, E.; et al. Circulating Tumour DNA Sequence Analysis as an Alternative to Multiple Myeloma Bone Marrow Aspirates. Nat. Commun. 2017, 8, 15086. [Google Scholar] [CrossRef]
- Vrabel, D.; Sedlarikova, L.; Besse, L.; Rihova, L.; Bezdekova, R.; Almasi, M.; Kubaczkova, V.; Brožová, L.; Jarkovsky, J.; Plonkova, H.; et al. Dynamics of Tumor-specific CfDNA in Response to Therapy in Multiple Myeloma Patients. Eur. J. Haematol. 2020, 104, 190–197. [Google Scholar] [CrossRef]
- Mithraprabhu, S.; Morley, R.; Khong, T.; Kalff, A.; Bergin, K.; Hocking, J.; Savvidou, I.; Bowen, K.M.; Ramachandran, M.; Choi, K.; et al. Monitoring Tumour Burden and Therapeutic Response through Analysis of Circulating Tumour DNA and Extracellular RNA in Multiple Myeloma Patients. Leukemia 2019, 33, 2022–2033. [Google Scholar] [CrossRef]
- Deshpande, S.; Tytarenko, R.G.; Wang, Y.; Boyle, E.M.; Ashby, C.; Schinke, C.D.; Thanendrarajan, S.; Zangari, M.; Zhan, F.; Davies, F.E.; et al. Monitoring Treatment Response and Disease Progression in Myeloma with Circulating Cell-free DNA. Eur. J. Haematol. 2021, 106, 230–240. [Google Scholar] [CrossRef]
- Manzoni, M.; Pompa, A.; Fabris, S.; Pelizzoni, F.; Ciceri, G.; Seia, M.; Ziccheddu, B.; Bolli, N.; Corradini, P.; Baldini, L.; et al. Limits and Applications of Genomic Analysis of Circulating Tumor DNA as a Liquid Biopsy in Asymptomatic Forms of Multiple Myeloma. HemaSphere 2020, 4, e402. [Google Scholar] [CrossRef]
- Pugh, T.J. Circulating Tumour DNA for Detecting Minimal Residual Disease in Multiple Myeloma. Semin. Hematol. 2018, 55, 38–40. [Google Scholar] [CrossRef]
- Mazzotti, C.; Buisson, L.; Maheo, S.; Perrot, A.; Chretien, M.-L.; Leleu, X.; Hulin, C.; Manier, S.; Hébraud, B.; Roussel, M.; et al. Myeloma MRD by Deep Sequencing from Circulating Tumor DNA Does Not Correlate with Results Obtained in the Bone Marrow. Blood Adv. 2018, 2, 2811–2813. [Google Scholar] [CrossRef] [Green Version]
- Long, X.; Xu, Q.; Lou, Y.; Li, C.; Gu, J.; Cai, H.; Wang, D.; Xu, J.; Li, T.; Zhou, X.; et al. The Utility of Non-invasive Liquid Biopsy for Mutational Analysis and Minimal Residual Disease Assessment in Extramedullary Multiple Myeloma. Br. J. Haematol. 2020, 189, e45–e48. [Google Scholar] [CrossRef] [Green Version]
- Manier, S.; Park, J.; Capelletti, M.; Bustoros, M.; Freeman, S.S.; Ha, G.; Rhoades, J.; Liu, C.J.; Huynh, D.; Reed, S.C.; et al. Whole-Exome Sequencing of Cell-Free DNA and Circulating Tumor Cells in Multiple Myeloma. Nat. Commun. 2018, 9, 1691. [Google Scholar] [CrossRef]
- Guo, G.; Raje, N.S.; Seifer, C.; Kloeber, J.; Isenhart, R.; Ha, G.; Yee, A.J.; O’Donnell, E.K.; Tai, Y.-T.; Richardson, P.G.; et al. Genomic Discovery and Clonal Tracking in Multiple Myeloma by Cell-Free DNA Sequencing. Leukemia 2018, 32, 1838–1841. [Google Scholar] [CrossRef] [PubMed]
- Waldschmidt, J.M.; Vijaykumar, T.; Knoechel, B.; Lohr, J.G. Tracking Myeloma Tumor DNA in Peripheral Blood. Best Pract. Res. Clin. Haematol. 2020, 33, 101146. [Google Scholar] [CrossRef]
- Mithraprabhu, S.; Spencer, A. Circulating Tumour DNA Analysis in Multiple Myeloma. Oncotarget 2017, 8, 90610–90611. [Google Scholar] [CrossRef] [PubMed]
- Medina Diaz, I.; Nocon, A.; Mehnert, D.H.; Fredebohm, J.; Diehl, F.; Holtrup, F. Performance of Streck CfDNA Blood Collection Tubes for Liquid Biopsy Testing. PLoS ONE 2016, 11, e0166354. [Google Scholar] [CrossRef] [PubMed]
- Franczak, C.; Filhine-Tresarrieu, P.; Gilson, P.; Merlin, J.-L.; Au, L.; Harlé, A. Technical Considerations for Circulating Tumor DNA Detection in Oncology. Expert Rev. Mol. Diagn. 2019, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
| Target | Methods | Cohort Size/Disease Stage | Evidence: Key Points | Application | Ref. |
|---|---|---|---|---|---|
| Somatic mutations | NGS ddPCR | n = 107 patients (33 PV, 56 ET, 14 PMF, and 4 uMPNs) |
| Concordance Response assessment | Garcia-Gisbert, et al., 2021 [78] |
| Target | Methods | Cohort Size/Disease Stage | Evidence: Key Points | Application | Ref. |
|---|---|---|---|---|---|
| IgH/TCR rearrangements | RQ-PCR | Precursor B-ALL, n = 62 T-ALL, n = 22 Diagnosis and post-treatment |
| Concordance Response assessment | van der Velden et al., 2002 [85] |
| IgH/TCR rearrangements | RQ-PCR in plasma vs. leucocytes | n = 21 (2–16 years) Diagnosis and post-treatment |
| Concordance | Schwarz et al., 2009 [86] |
| IgH/TCR rearrangements | RQ-PCR vs. flow cytometry | n = 206 Diagnosis and post-treatment |
| Response assessment by MRD Prognostication | Cheng et al., 2013 [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colmenares, R.; Álvarez, N.; Barrio, S.; Martínez-López, J.; Ayala, R. The Minimal Residual Disease Using Liquid Biopsies in Hematological Malignancies. Cancers 2022, 14, 1310. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14051310
Colmenares R, Álvarez N, Barrio S, Martínez-López J, Ayala R. The Minimal Residual Disease Using Liquid Biopsies in Hematological Malignancies. Cancers. 2022; 14(5):1310. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14051310
Chicago/Turabian StyleColmenares, Rafael, Noemí Álvarez, Santiago Barrio, Joaquín Martínez-López, and Rosa Ayala. 2022. "The Minimal Residual Disease Using Liquid Biopsies in Hematological Malignancies" Cancers 14, no. 5: 1310. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers14051310