Simple and Facile Fabrication of Anion-Vacancy-Induced MoO3−X Catalysts for Enhanced Hydrogen Evolution Activity
Abstract
:1. Introduction
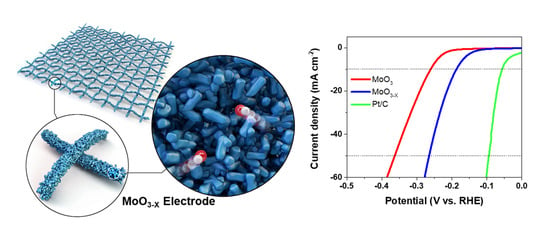
2. Results
3. Materials and Methods
3.1. Preparations of Materials
3.2. Material Characterization
3.3. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2014, 414, 332–337. [Google Scholar] [CrossRef]
- Schlapbach, L. Hydrogen-fuelled vehicles. Nature 2009, 406, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.A. A realizable renewable energy future. Science 1999, 285, 687–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D transition-metal-dichalcogenide-nanosheet-based composites for photocatalytic and electrocatalytic hydrogen evolution reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.P.; Guo, C.; Zheng, Y.; Qiao, S.Z. Surface and interface engineering of noble-metal-free electrocatalysts for efficient energy conversion processes. Acc. Chem. Res. 2017, 50, 915–923. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. advancing the electrochemistry of the hydrogen evolution reaction through combining experiment and theory. Angew. Chem. 2015, 54, 52–65. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-engraved Co3O4 nanosheets with oxygen vacancies and high surface area for the oxygen evolution reaction. Angew. Chem. 2016, 55, 5277–5281. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Liu, D.; Zou, Y.; Wang, S. Water-plasma-enabled exfoliation of ultrathin layered double hydroxide nanosheets with multivacancies for water oxidation. Adv. Mater. 2017, 29, 1701546. [Google Scholar] [CrossRef]
- Kim, J.; Yin, X.; Tsao, K.-C.; Fang, S.; Yang, H. Ca2Mn2O5 as oxygen-deficient perovskite electrocatalyst for oxygen evolution reaction. J. Am. Chem. Soc. 2014, 136, 14646–14649. [Google Scholar] [CrossRef]
- Wu, M.; Ke, S.; Chen, W.; Zhang, S.; Zhu, M.; Zhang, Y.; Foo, M.L.; Tang, L. Optimization of the facet structure of cobalt oxide catalysts for enhanced hydrogen evolution reaction. Catal. Sci. Technol. 2020, 10, 1040–1047. [Google Scholar] [CrossRef]
- Lovell, E.; Lu, X.; Zhang, Q.; Scott, J.; Amal, R. From passivation to activation–tunable nickel/nickel oxide for hydrogen evolution electrocatalysis. Chem. Commun. 2020, 56, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.K.; Verma, M.; Sohn, Y.; Deshpande, P.A.; Pradhan, D. Highly active tungsten oxide nanoplate electrocatalysts for the hydrogen evolution reaction in acidic and near neutral electrolytes. ACS Omega 2017, 2, 7039–7047. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, C.; Zhang, Y.; Zheng, Q.; Zhang, S.; Mu, X.; Chen, C.; Ma, J.; Mu, S. Vacancy-coordinated hydrogen evolution reaction on MoO3−x anchored atomically dispersed MoRu pairs. J. Mater. Chem. A 2019, 7, 14466–14472. [Google Scholar] [CrossRef]
- Kim, H.; Cook, J.B.; Lin, H.; Ko, J.S.; Tolbert, S.H.; Ozolins, V.; Dunn, B. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3−x. Nat. Mater. 2017, 16, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhang, X.; Cheng, X.; Xu, Y.-M.; Gao, S.; Zhao, H.; Zhou, X.; Huo, L.-H. Oxygen-vacancy-enriched porous α-MoO3 nanosheets for trimethylamine sensing. ACS Appl. Nano Mater. 2019, 2, 8016–8026. [Google Scholar] [CrossRef]
- Yu, M.; Shao, H.; Wang, G.; Yang, F.; Liang, C.; Rozier, P.; Wang, C.-Z.; Lu, X.; Simon, P.; Feng, X. Interlayer gap widened α-phase molybdenum trioxide as high-rate anodes for dual-ion-intercalation energy storage devices. Nat. Commun. 2020, 11, 1348. [Google Scholar] [CrossRef] [Green Version]
- Brezesinski, T.; Wang, J.; Tolbert, S.H.; Dunn, B. Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 2010, 9, 146–151. [Google Scholar] [CrossRef]
- Yazdani, S.; Kashfi-Sadabad, R.; Huan, T.D.; Morales-Acosta, M.D.; Pettes, M.T. Polyelectrolyte-assisted oxygen vacancies: A new route to defect engineering in molybdenum oxide. Langmuir 2018, 34, 6296–6306. [Google Scholar] [CrossRef]
- Shi, X.-R.; Wang, J.; Hermann, K. Theoretical cluster studies on the catalytic sulfidation of MoO3. J. Phys. Chem. C 2010, 114, 6791–6801. [Google Scholar] [CrossRef]
- Borgschulte, A.; Sambalova, O.; Delmelle, R.; Jenatsch, S.; Hany, R.; Nüesch, F. Hydrogen reduction of molybdenum oxide at room temperature. Sci. Rep. 2017, 7, 40761. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.-L.; Zhao, S.-X.; Yu, L.; Zheng, X.-X.; Wang, Y.-F.; Yu, L.-Q.; Nan, C.-W.; Cao, G. Oxygen vacancy-enriched MoO3−x nanobelts for asymmetric supercapacitors with excellent room/low temperature performance. J. Mater. Chem. A 2019, 7, 13205–13214. [Google Scholar] [CrossRef]
- Luo, Z.; Miao, R.; Huan, T.D.; Mosa, I.M.; Poyraz, A.S.; Zhong, W.; Cloud, J.E.; Kriz, D.A.; Thanneeru, S.; He, J.; et al. Mesoporous MoO3-x material as an efficient electrocatalyst for hydrogen evolution reactions. Adv. Energy Mater. 2016, 6, 1600528. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, D.; Zheng, Y.; Vasileff, A.; Qiao, S.Z. Self-supported earth-abundant nanoarrays as efficient and robust electrocatalysts for energy-related reactions. ACS Catal. 2018, 8, 6707–6732. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, Y.-P.; Yuan, Z.-Y. Three-dimensional electrocatalysts for sustainable water splitting reactions. Eur. J. Inorg. Chem. 2016, 2016, 1916–1923. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148. [Google Scholar] [CrossRef]
- Kashfi-Sadabad, R.; Yazdani, S.; Huan, T.D.; Cai, Z.; Pettes, M.T. Role of oxygen vacancy defects in the electrocatalytic activity of substoichiometric molybdenum oxide. J. Phys. Chem. C 2018, 122, 18212–18222. [Google Scholar] [CrossRef]
- Oleksak, R.P.; Kapoor, M.; Perea, D.; Holcomb, G.R.; Doğan, Ö.N. The role of metal vacancies during high-temperature oxidation of alloys. NPJ Mater. Degrad. 2018, 2, 25. [Google Scholar] [CrossRef]
- Yamashita, T.; Yokoyama, H. Molybdenum anode: A novel electrode for enhanced power generation in microbial fuel cells, identified via extensive screening of metal electrodes. Biotechnol. Biofuels 2018, 11, 39. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zheng, C.; Wang, S.; Hou, Y. 3D arrays of molybdenum sulphide nanosheets on Mo meshes: Efficient electrocatalysts for hydrogen evolution reaction. Electrochim. Acta 2015, 174, 653–659. [Google Scholar] [CrossRef]
- Manthiram, K.; Alivisatos, A.P. Tunable localized surface plasmon resonances in tungsten oxide nanocrystals. J. Am. Chem. Soc. 2012, 134, 3995–3998. [Google Scholar] [CrossRef]
- Dieterle, M.; Weinberg, G.; Mestl, G. Raman spectroscopy of molybdenum oxides. Phys. Chem. Chem. Phys. 2002, 4, 812–821. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2Nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, R.S.; Haque, F.; Mohiuddin, M.; Carey, B.J.; Syed, N.; Zavabeti, A.; Zhang, B.; Khan, H.; Berean, K.J.; Ou, J.Z.; et al. Highly active two dimensional α-MoO3−x for the electrocatalytic hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 24223–24231. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, S.; Lee, Y.-W.; Hong, J.; Sohn, J.I. Simple and Facile Fabrication of Anion-Vacancy-Induced MoO3−X Catalysts for Enhanced Hydrogen Evolution Activity. Catalysts 2020, 10, 1180. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10101180
Jo S, Lee Y-W, Hong J, Sohn JI. Simple and Facile Fabrication of Anion-Vacancy-Induced MoO3−X Catalysts for Enhanced Hydrogen Evolution Activity. Catalysts. 2020; 10(10):1180. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10101180
Chicago/Turabian StyleJo, Seunghwan, Young-Woo Lee, John Hong, and Jung Inn Sohn. 2020. "Simple and Facile Fabrication of Anion-Vacancy-Induced MoO3−X Catalysts for Enhanced Hydrogen Evolution Activity" Catalysts 10, no. 10: 1180. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10101180