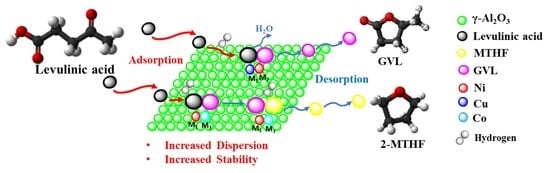
Supported Bimetallic Catalysts for the Solvent-Free Hydrogenation of Levulinic Acid to γ-Valerolactone: Effect of Metal Combination (Ni-Cu, Ni-Co, Cu-Co)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Catalytic Activity
2.2.1. Effect of Metal Ratio and Combination
2.2.2. Effect of Reaction Temperature
2.2.3. Effect of Reaction Pressure
2.2.4. Effect of Reaction Time
2.2.5. Effect of Catalyst Loading
2.2.6. Metal Leaching
2.3. Comparison with Previous Literature
3. Experimental
3.1. Materials
3.2. Methods
3.2.1. Catalyst Synthesis
Preparation of γ-Al2O3 Nanofibers
Catalyst Preparation
3.2.2. Catalyst Characterization
3.2.3. Activity Tests
Hydrogenation of Levulinic Acid
Product Qualification and Quantification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Bozell, J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “top 10” revisited. Green Chem. 2010, 12, 539–555. [Google Scholar] [CrossRef]
- Pileidis, F.D.; Titirici, M. Levulinic Acid Biorefineries: New Challenges for Efficient Utilization of Biomass. ChemSusChem 2016, 9, 562–582. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Yu, I.K.M.; Tsang, D.C.W.; Ng, Y.H.; Ok, Y.S.; Sherwood, J.; Clark, J.H. Green synthesis of gamma-valerolactone (GVL) through hydrogenation of biomass-derived levulinic acid using non-noble metal catalysts: A critical review. Chem. Eng. J. 2019, 372, 992–1006. [Google Scholar] [CrossRef]
- Horvath, I.T.; Mehdi, H.; Fabos, V.; Boda, L.; Mika, L.T. γ-Valerolactone — a sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Abdelrahman, O.A.; Heyden, A.; Bond, J.Q. Analysis of kinetics and reaction pathways in the aqueous-phase hydrogenation of levulinic acid to form γ-Valerolactone over Ru/C. ACS Catal. 2014, 4, 1171–1181. [Google Scholar] [CrossRef]
- Yan, Z.; Lin, L.; Liu, S. Synthesis of γ -Valerolactone by Hydrogenation of Biomass-derived Levulinic Acid over Ru/C Catalyst. Energy Fuels 2009, 23, 3853–3858. [Google Scholar] [CrossRef]
- Upare, P.P.; Lee, J.M.; Hwang, D.W.; Halligudi, S.B.; Hwang, Y.K.; Chang, J.S. Selective hydrogenation of levulinic acid to γ-valerolactone over carbon-supported noble metal catalysts. J. Ind. Eng. Chem. 2011, 17, 287–292. [Google Scholar] [CrossRef]
- Putro, J.N.; Kurniawan, A.; Soetaredjo, F.E.; Lin, S.S.; Ju, Y.; Ismadji, S. Production of gamma-valerolactone from and acid-activated bentonite as a co-catalyst. RSC Adv. 2015, 5, 41285–41299. [Google Scholar] [CrossRef] [Green Version]
- Yan, K.; Lafleur, T.; Jarvis, C.; Wu, G. Clean and selective production of γ-valerolactone from biomass-derived levulinic acid catalyzed by recyclable Pd nanoparticle catalyst. J. Clean. Prod. 2014, 72, 230–232. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Hasan, M.A. Pd/C catalyzed conversion of levulinic acid to γ-valerolactone using alcohol as a hydrogen donor under microwave conditions. CATCOM 2015, 60, 5–7. [Google Scholar] [CrossRef]
- Gupta, S.S.R.; Kantam, M.L. Selective hydrogenation of levulinic acid into γ-valerolactone over Cu/Ni hydrotalcite-derived catalyst. Catal. Today 2018, 309, 189–194. [Google Scholar] [CrossRef]
- Obregón, I.; Corro, E.; Izquierdo, U.; Requies, J.; Arias, P.L. Levulinic acid hydrogenolysis on Al2O3-based Ni-Cu bimetallic catalysts. Chin. J. Catal. 2014, 35, 656–662. [Google Scholar] [CrossRef]
- Sun, D.; Ohkubo, A.; Asami, K.; Katori, T.; Yamada, Y.; Sato, S. Vapor-phase hydrogenation of levulinic acid and methyl levulinate to γ-valerolactone over non-noble metal-based catalysts. Mol. Catal. 2017, 437, 105–113. [Google Scholar] [CrossRef]
- Long, X.; Sun, P.; Li, Z.; Lang, R.; Xia, C.; Li, F. Magnetic Co/Al 2 O 3 catalyst derived from hydrotalcite for hydrogenation of levulinic acid to γ-valerolactone. Chin. J. Catal. 2015, 36, 1512–1518. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Fan, G.; Yang, L.; Li, F. Hierarchical Flower-like Bimetallic NiCu catalysts for Catalytic Transfer Hydrogenation of Ethyl Levulinate into γ-Valerolactone. Ind Eng. Chem Res. 2019, 58, 10317–10327. [Google Scholar] [CrossRef]
- Torres, D.; Pinilla, J.L.; Suelves, I. Co-, Cu-and Fe-doped Ni/Al2O3 catalysts for the catalytic decomposition of methane into hydrogen and carbon nanofibers. Catalysts 2018, 8, 300. [Google Scholar] [CrossRef] [Green Version]
- Sharifi, M.; Haghighi, M.; Rahmani, F.; Rahemi, N. Reforming of Biogas over Co- and Cu-Promoted Ni/Al2O3-ZrO2 Nanocatalyst Synthesized via Sequential Impregnation Method. J. Renew. Energy Environ. 2014, 1, 53–63. [Google Scholar]
- Xue, H.; Xu, J.; Gong, X.; Hu, R. Performance of a Ni-Cu-Co/Al2O3 Catalyst on in-situ Hydrodeoxygenation of Bio-derived Phenol. Catalysts 2019, 9, 952. [Google Scholar] [CrossRef] [Green Version]
- Shejale, A.D.; Yadav, G.D. Ni-Cu and Ni-Co Supported on La-Mg Based Metal Oxides Prepared by Coprecipitation and Impregnation for Superior Hydrogen Production via Steam Reforming of Glycerol. Ind. Eng. Chem. Res. 2018, 57, 4785–4797. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Yang, Z.; Liang, T.; Liu, S.; Zhou, Z.; Li, X. Bimetallic Ni-M (M = Co, Cu, and Zn) supported on attapulgite as catalysts for hydrogen production from glycerol steam reforming. Appl. Catal. A Gen. 2018, 550, 214–227. [Google Scholar] [CrossRef]
- You, X.; Wang, X.; Ma, Y.; Liu, J.; Liu, W.; Xu, X.; Peng, H.; Li, C.; Zhou, W.; Yuan, P.; et al. Ni-Co/Al2O3 Bimetallic Catalysts for CH4 Steam Reforming: Elucidating the Role of Co for Improving Coke Resistance. ChemCatChem 2014, 6, 3377–3386. [Google Scholar] [CrossRef]
- Srivastava, S.; Jadeja, G.C.; Parikh, J. A versatile bi-metallic copper-cobalt catalyst for liquid-phase hydrogenation of furfural to 2-methylfuran. RSC Adv. 2016, 6, 1649. [Google Scholar] [CrossRef]
- Novodárszki, G.; Solt, H.E.; Valyon, J.; Lónyi, F.; Hancsók, J.; Deka, D.; Tuba, R.; Mihalyi, M.R. Selective hydroconversion of levulinic acid to γ-valerolactone or 2-methyltetrahydrofuran over silica-supported cobalt catalysts. Catal. Sci. Technol. 2019, 9, 2291–2304. [Google Scholar]
- Xue, Z.; Liu, Q.; Wang, J.; Mu, T. Valorization of levulinic acid over non-noble metal catalysts: Challenges and opportunities. Green Chem. 2018, 20, 4391–4408. [Google Scholar] [CrossRef]
- Yu, Z.; Meng, F.; Wang, Y.; Sun, Z.; Liu, Y.; Shi, C.; Wang, W.; Wang, A. Catalytic Transfer Hydrogenation of Levulinic Acid to γ-Valerolactone over Ni3P-CePO4Catalysts. Ind. Eng. Chem. Res. 2020, 59, 7416–7425. [Google Scholar] [CrossRef]
- Sun, D.; Takahashi, Y.; Yamada, Y.; Sato, S. Efficient formation of angelica lactones in a vapor-phase conversion of levulinic acid. Appl. Catal. A Gen. 2016, 526, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Al-Shaal, M.G.; Dzierbinski, A.; Palkovits, R. Solvent-free γ-valerolactone hydrogenation to 2-methyltetrahydrofuran catalyzed by Ru/C: A reaction network analysis. Green Chem. 2014, 16, 1358–1364. [Google Scholar] [CrossRef]
- Wu, J.; Gao, G.; Sun, P.; Long, X.; Li, F. Synergetic Catalysis of Bimetallic CuCo Nanocomposites for Selective Hydrogenation of Bio derived Esters. ACS Catal. 2017, 7, 7890–7901. [Google Scholar] [CrossRef]
- Obregon, I.; Gandarias, I.; Mileti, N.; Ocio, A.; Arias, P.L. One-Pot 2-Methyltetrahydrofuran Production from Levulinic Acid in Green Solvents Using Ni-Cu/Al2O3 Catalysts. ChemSusChem 2015, 8, 3483–3488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mao, J.; Li, S.; Yin, J.; Sun, X.; Guo, X.; Song, C.; Zhou, J. Hydrogenation of levulinic acid into gamma-valerolactone over in situ reduced CuAg bimetallic catalyst: Strategy and mechanism of preventing Cu leaching. Appl. Catal. B Environ. 2018, 232, 1–10. [Google Scholar] [CrossRef]
- Srivastava, S.; Jadeja, G.C.; Parikh, J. Copper-cobalt catalyzed liquid phase hydrogenation of furfural to 2-methylfuran: An optimization, kinetics, and reaction mechanism study. Chem. Eng. Res. Des. 2018, 132, 313–324. [Google Scholar] [CrossRef]
- Van Slagmaat, C.A.M.R.; Delgove, M.A.F.; Stouten, J.; Morick, L.; Van Der Meer, Y.; Bernaerts, K.V.; De Wildeman, S.M.A. Solvent-free hydrogenation of levulinic acid to γ-valerolactone using a Shvo catalyst precursor: Optimization, thermodynamic insights, and life cycle assessment. Green Chem. 2020, 22, 2443–2458. [Google Scholar] [CrossRef] [Green Version]
- Xiao, C.; Goh, T.W.; Qi, Z.; Goes, S.; Brashler, K.; Perez, C.; Huang, W. Conversion of Levulinic Acid to γ-Valerolactone over Few-Layer Graphene-Supported Ruthenium Catalysts. ACS Catal. 2016, 6, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Amenuvor, G.; Makhubela, B.C.E.; Darkwa, J. Efficient solvent-free hydrogenation of levulinic acid to γ-valerolactone by pyrazolylphosphite and pyrazolylphosphinite ruthenium (II) complexes. ACS Sustain. Chem. Eng. 2016, 4, 6010–6018. [Google Scholar] [CrossRef]
- Hengst, K.; Schubert, M.; Carvalho, H.W.P.; Lu, C.; Kleist, W.; Grunwaldt, J.D. Synthesis of γ-valerolactone by hydrogenation of levulinic acid over supported nickel catalysts. Appl. Catal. A Gen. 2015, 502, 18–26. [Google Scholar] [CrossRef]
- Liu, C.; Hong, J.; Zhang, Y.; Zhao, Y.; Wang, L.; Wei, L.; Chen, S.; Wang, G.; Li, J. Synthesis of γ-Al2O3 nanofibers stabilized Co3O4 nanoparticles as highly active and stable Fischer-Tropsch synthesis catalysts. Fuel 2016, 180, 777–784. [Google Scholar] [CrossRef]
- Jiao, H.; Zhao, X.; Lv, C.; Wang, Y.; Yang, D.; Li, Z.; Yao, X. Nb2O5-γ-Al2O3 nanofibers as heterogeneous catalysts for efficient conversion of glucose to 5-hydroxymethylfurfural. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]













| Catalysts | Ni (wt.%) a | Cu (wt.%) a | Co (wt.%) a | Total wt.% | M1/M2 | SBET (m2/g) b | Pore Volume (cm3/g) c | Pore Diameter (nm) c | Crystallize size (nm) d |
|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 259.0 | 0.99 | 17.6 | 24.6 |
| Ni/Al2O3 | 37.3 | 0.0 | 0.0 | 37.3 | 0.0 | 129.6 | 0.29 | 7.4 | 41.7 |
| Cu/Al2O3 | 0.0 | 31.0 | 0.0 | 31.0 | 0.0 | 147.0 | 0.53 | 14.2 | 82.8 |
| Co/Al2O3 | 0.0 | 0.0 | 32.3 | 34.1 | 0.0 | 152.3 | 0.48 | 13.9 | 32.8 |
| [2:1] Ni-Cu/Al2O3 | 20.9 | 12.1 | 0.0 | 33.0 | 1.72 | 144.0 | 0.38 | 11.8 | 58.9 |
| [2:1] Ni-Co/Al2O3 | 21.4 | 0.0 | 11.3 | 32.7 | 1.89 | 159.4 | 0.45 | 12.0 | 36.3 |
| [2:1] Co-Cu/Al2O3 | 0.0 | 23.1 | 13.7 | 36.8 | 1.69 | 162.1 | 0.41 | 13.3 | 63.1 |
| Solvent-Free Hydrogenation of LA to GVL | ||||||
|---|---|---|---|---|---|---|
| Catalysts | Temp. (°C) | H2 Pressure (bar) | Time (h) | LA Conv. (%) | GVL Yield (%) | Ref. |
| Commercial Ru/C | 190 | 12 | 0.75 | 100 | 100 | [29] |
| Shvo catalyst (Ru-based) | 100 | 50 | 5 | 100 | 99.5 | [34] |
| Ru/FLG | 37 | 40 | 58 | 100 | 93.5 | [35] |
| Pyrazolylphosphite and Pyrazolylphosphinite Ru (II) complexes | 110 | 15 | 12 | 100 | 100 | [36] |
| Co/SiO2 | 200 | 30 | 10 (20) | 100 | 80 (98) | [25] |
| Ni/Al2O3 | 200 | 50 | 4 | 92 | 92 | [37] |
| [2:1] Ni-Co/Al2O3 | 220 | 30 | 6 | 100 | 83 | This work |
| [2:1] Ni-Cu/Al2O3 | 220 | 30 | 6 | 100 | >99 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebresillase, M.N.; Raguindin, R.Q.; Kim, H.; Seo, J.G. Supported Bimetallic Catalysts for the Solvent-Free Hydrogenation of Levulinic Acid to γ-Valerolactone: Effect of Metal Combination (Ni-Cu, Ni-Co, Cu-Co). Catalysts 2020, 10, 1354. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10111354
Gebresillase MN, Raguindin RQ, Kim H, Seo JG. Supported Bimetallic Catalysts for the Solvent-Free Hydrogenation of Levulinic Acid to γ-Valerolactone: Effect of Metal Combination (Ni-Cu, Ni-Co, Cu-Co). Catalysts. 2020; 10(11):1354. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10111354
Chicago/Turabian StyleGebresillase, Mahlet N., Reibelle Q. Raguindin, Hern Kim, and Jeong Gil Seo. 2020. "Supported Bimetallic Catalysts for the Solvent-Free Hydrogenation of Levulinic Acid to γ-Valerolactone: Effect of Metal Combination (Ni-Cu, Ni-Co, Cu-Co)" Catalysts 10, no. 11: 1354. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10111354