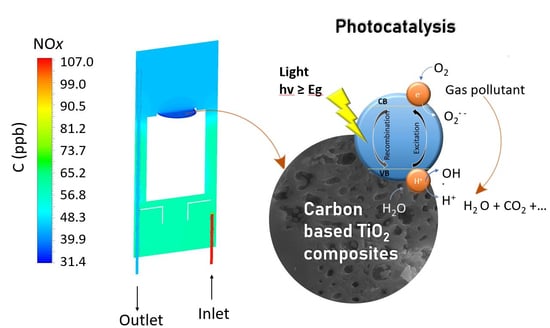
Experimental and Computational Analysis of NOx Photocatalytic Abatement Using Carbon-Modified TiO2 Materials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Experimental Results
2.2. Flow Field Simulation
2.3. PCO Analysis with the Conventionally Estimated L-N Model Parameters
2.3.1. Determination of the L-H Model Parameters from the Experimental Data
2.3.2. Results of the PCO Analysis with the Conventionally Estimated L-N Model Parameters
2.4. Kinetic Analysis with the CFD Modified L-N Model Parameters
3. Materials and Methods
3.1. Materials Synthesis
3.2. Materials Characterization
3.3. Experimental Setup
Photocatalytic Reactor Layout
3.4. Kinetic Modeling of Photocatalytic Degradation
3.4.1. Langmuir–Hinshelwood Mechanism
3.4.2. Identification of L-H Model Coefficients by Using the Experimental Results
3.5. CFD Analysis
3.5.1. Flow Modeling
3.5.2. Solver Parameters
3.5.3. Meshing and Boundary Conditions
3.5.4. Modeling and Simulation of NOx Transport
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wainman, T.; Weschler, C.J.; Lioy, P.J.; Zhang, J. Effects of Surface Type and Relative Humidity on the Production and Concentration of Nitrous Acid in a Model Indoor Environment. Environ. Sci. Technol. 2001, 35, 2200–2206. [Google Scholar] [CrossRef] [PubMed]
- European Environment Agency. Air Quality in Europe—2019 Report; European Environment Agency: Copenhagen, Denmark, 2019; ISBN 978-92-9480-088-6.
- WHO. WHO Guidelines for Indoor Air Quality: Selected Pollutants; WHO: Geneva, Switzerland, 2010; p. 454. [Google Scholar]
- Salonen, H.; Salthammer, T.; Morawska, L. Human exposure to NO2 in school and office indoor environments. Environ. Int. 2019, 130, 104887. [Google Scholar] [CrossRef] [PubMed]
- Sterling, T.D.; Dimich, H.; Kobayashi, D. Indoor Byproduct Levels of Tobacco Smoke: A Critical Review of the Literature. J. Air Pollut. Control Assoc. 1982, 32, 250–259. [Google Scholar] [CrossRef] [Green Version]
- Ângelo, J.; Andrade, L.; Madeira, L.M.; Mendes, A. An overview of photocatalysis phenomena applied to NOx abatement. J. Environ. Manag. 2013, 129, 522–539. [Google Scholar] [CrossRef] [PubMed]
- Lasek, J.; Yu, Y.-H.; Wu, J.C.S. Removal of NOx by photocatalytic processes. J. Photochem. Photobiol. C Photochem. Rev. 2013, 14, 29–52. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Pirola, C.; Galli, F.; Cerrato, G.; Morandi, S.; Capucci, V. Pigmentary TiO2: A challenge for its use as photocatalyst in NOx air purification. Chem. Eng. J. 2015, 261, 76–82. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Pirola, C.; Galli, F.; Vitali, S.; Minguzzi, A.; Stucchi, M.; Manenti, F.; Capucci, V. NOx degradation in a continuous large-scale reactor using full-size industrial photocatalytic tiles. Catal. Sci. Technol. 2016, 6, 2261–2267. [Google Scholar] [CrossRef] [Green Version]
- Devahasdin, S.; Fan, C.; Li, K.; Chen, D.H. TiO2 photocatalytic oxidation of nitric oxide: Transient behavior and reaction kinetics. J. Photochem. Photobiol. A Chem. 2003, 156, 161–170. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Tucci, A.; Škapin, A.S.; Esposito, L. Effects of SiO2 addition on TiO2 crystal structure and photocatalytic activity. J. Eur. Ceram. Soc. 2010, 30, 2481–2490. [Google Scholar] [CrossRef]
- Giampiccolo, A.; Tobaldi, D.M.; Leonardi, S.G.; Murdoch, B.J.; Seabra, M.P.; Ansell, M.P.; Neri, G.; Ball, R.J. Sol gel graphene/TiO2 nanoparticles for the photocatalytic-assisted sensing and abatement of NO2. Appl. Catal. B Environ. 2019, 243, 183–194. [Google Scholar] [CrossRef]
- Rozman, N.; Tobaldi, D.M.; Cvelbar, U.; Puliyalil, H.; Labrincha, J.A.; Legat, A.; Škapin, A.S. Hydrothermal synthesis of rare-earth modified titania: Influence on phase composition, optical properties, and photocatalytic activity. Materials 2019, 12, 713. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.R.F.; Lourenço, M.A.O.; Tobaldi, D.M.; da Silva, C.F.; Seabra, M.P.; Ferreira, P. Carbon-modified titanium oxide materials for photocatalytic water and air decontamination. Chem. Eng. J. 2020, 387, 124099. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Tseng, Y.-H.; Huang, J.-H.; Chao, C.C.; Chen, C.-C.; Wang, I. Photocatalytic Activity for Degradation of Nitrogen Oxides over Visible Light Responsive Titania-Based Photocatalysts. Environ. Sci. Technol. 2006, 40, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Saeli, M.; Tobaldi, D.M.; Rozman, N.; Škapin, A.S.; Labrincha, J.A.; Pullar, R.C. Photocatalytic nano-composite architectural lime mortar for degradation of urban pollutants under solar and visible (interior) light. Constr. Build. Mater. 2017, 152, 206–213. [Google Scholar] [CrossRef]
- Chen, X.; Burda, C. The Electronic Origin of the Visible-Light Absorption Properties of C-, N- and S-Doped TiO2 Nanomaterials. J. Am. Chem. Soc. 2008, 130, 5018–5019. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Wasada, K.; Osaki, M.; Shono, E.; Adachi, K.; Toukai, N.; Kominami, H.; Kera, Y. Photocatalytic oxidation of nitrogen oxide over titania–zeolite composite catalyst to remove nitrogen oxides in the atmosphere. Appl. Catal. B Environ. 2001, 30, 429–436. [Google Scholar] [CrossRef]
- Ballari, M.M.; Hunger, M.; Hüsken, G.; Brouwers, H.J.H. NOx photocatalytic degradation employing concrete pavement containing titanium dioxide. Appl. Catal. B Environ. 2010, 95, 245–254. [Google Scholar] [CrossRef]
- Hunger, M.; Hüsken, G.; Brouwers, H.J.H. Photocatalytic degradation of air pollutants—From modeling to large scale application. Cem. Concr. Res. 2010, 40, 313–320. [Google Scholar] [CrossRef]
- Yu, Q.L.; Ballari, M.M.; Brouwers, H.J.H. Indoor air purification using heterogeneous photocatalytic oxidation. Part II: Kinetic study. Appl. Catal. B Environ. 2010, 99, 58–65. [Google Scholar] [CrossRef]
- Muñoz, V.; Casado, C.; Suárez, S.; Sánchez, B.; Marugán, J. Photocatalytic NOx removal: Rigorous kinetic modelling and ISO standard reactor simulation. Catal. Today 2019, 326, 82–93. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Sun, H.; Liu, J.; Pareek, V.K.; Wang, S. A review on photocatalysis for air treatment: From catalyst development to reactor design. Chem. Eng. J. 2017, 310, 537–559. [Google Scholar] [CrossRef]
- Mohseni, M.; Taghipour, F. Experimental and CFD analysis of photocatalytic gas phase vinyl chloride (VC) oxidation. Chem. Eng. Sci. 2004, 59, 1601–1609. [Google Scholar] [CrossRef]
- Denny, F.; Scott, J.; Pareek, V.; Peng, G.D.; Amal, R. CFD modelling for a TiO2-coated glass-bead photoreactor irradiated by optical fibres: Photocatalytic degradation of oxalic acid. Chem. Eng. Sci. 2009, 64, 1695–1706. [Google Scholar] [CrossRef]
- Queffeulou, A.; Geron, L.; Schaer, E. Prediction of photocatalytic air purifier apparatus performances with a CFD approach using experimentally determined kinetic parameters. Chem. Eng. Sci. 2010, 65, 5067–5074. [Google Scholar] [CrossRef]
- Chong, S.; Wang, S.; Tadé, M.; Ang, H.M.; Pareek, V. Simulations of photodegradation of toluene and formaldehyde in a monolith reactor using computational fluid dynamics. AIChE J. 2011, 57, 724–734. [Google Scholar] [CrossRef]
- Passalía, C.; Alfano, O.M.; Brandi, R.J. Optimal Design of a Corrugated-Wall Photocatalytic Reactor Using Efficiencies in Series and Computational Fluid Dynamics (CFD) Modeling. Ind. Eng. Chem. Res. 2013, 52, 6916–6922. [Google Scholar] [CrossRef]
- de OB Lira, J.; Padoin, N.; Vilar, V.J.P.; Soares, C. Photocatalytic NOx abatement: Mathematical modeling, CFD validation and reactor analysis. J. Hazard. Mater. 2019, 372, 145–153. [Google Scholar] [CrossRef]
- Verbruggen, S.W.; Lenaerts, S.; Denys, S. Analytic versus CFD approach for kinetic modeling of gas phase photocatalysis. Chem. Eng. J. 2015, 262, 1–8. [Google Scholar] [CrossRef]
- Einaga, H.; Tokura, J.; Teraoka, Y.; Ito, K. Kinetic analysis of TiO2-catalyzed heterogeneous photocatalytic oxidation of ethylene using computational fluid dynamics. Chem. Eng. J. 2015, 263, 325–335. [Google Scholar] [CrossRef]
- Nakahara, K.; Muttakin, M.; Yamamoto, K.; Ito, K. Computational fluid dynamics modelling of the visible light photocatalytic oxidation process of toluene for indoor building materials with locally doped titanium dioxide. Indoor Built Environ. 2019, 1420326X19854499. [Google Scholar] [CrossRef]
- Roegiers, J.; van Walsem, J.; Denys, S. CFD- and radiation field modeling of a gas phase photocatalytic multi-tube reactor. Chem. Eng. J. 2017. [Google Scholar] [CrossRef]
- Nakahara, K.; Yamaguchi, T.; Lim, E.; Ito, K. Computational fluid dynamics modeling and parameterization of the visible light photocatalytic oxidation process of toluene for indoor building material. Sustain. Cities Soc. 2017, 35, 298–308. [Google Scholar] [CrossRef]
- Franses, E.I. Thermodynamics with Chemical Engineering Applications; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Ranade, V.V. Computational Flow Modeling for Chemical Reactor Engineering; Elsevier Science Publishing Co Inc.: San Diego, CA, USA, 2002. [Google Scholar]
- Ollis, D.F. Kinetics of Photocatalyzed Reactions: Five Lessons Learned. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kecili, R.; Hussain, C.M. Chapter 4—Mechanism of Adsorption on Nanomaterials. In Nanomaterials in Chromatography; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 89–115. [Google Scholar] [CrossRef]
- ANSYS. ANSYS Fluent Theory Guide 19.0; ANSYS, Inc.: Canonsburg, PA, USA, 2018; Volume Version19.0. [Google Scholar]
- Launder, B.E.; Spalding, D.B. The numerical computation of turbulent flows. Comput. Methods Appl. Mech. Eng. 1974, 3, 269–289. [Google Scholar] [CrossRef]
- ANSYS. ANSYS Fluent User’s Guide 19.0; ANSYS, Inc.: Canonsburg, PA, USA, 2018; Volume Version19.0. [Google Scholar]









| Material | Cin (ppb) | Cout (ppb) | Conversion (%) |
|---|---|---|---|
| T300_GO | 107 | 44 | 58.9 |
| 192 | 76 | 60.4 | |
| 425 | 175 | 58.8 | |
| 608 | 253 | 58.4 | |
| 787 | 353 | 55.2 | |
| T300_CNT | 108 | 45 | 58.3 |
| 195 | 74 | 62.1 | |
| 420 | 167 | 60.2 | |
| 618 | 253 | 59.1 | |
| 805 | 330 | 59.0 | |
| P25 | 197 | 76 | 61.4 |
| Materials | k’(kg/m2s) | Kp.(m3/kg) |
|---|---|---|
| T300_GO | 4.02 × 10−8 | 1.05 × 105 |
| T300_CNT | 4.92 × 10−8 | 8.75 × 104 |
| Materials | k’(kg/m2s) | Kp.(m3/kg) |
|---|---|---|
| T300_GO | 7.25 × 10−8 | 1.89 × 105 |
| T300_CNT | 9.04 × 10−8 | 1.61 × 105 |
| Turbulence Model | K-ε Model, Realizable, Standard Wall Function |
|---|---|
| Scheme | Convection term: Second-Order Upwind |
| Inlet Boundary | D = 0.004 m; Ti = 1 wt. %; Mass Flow Rate (MFR) = 2.06208 × 10−5 (kg/s) |
| Outlet Boundary | Outlet boundary D = 0.004 m; MFR = 2.06208 × 10−5 (kg/s) |
| Fan Boundary Condition | lump parameter model/polynomial pressure jump across the fan |
| Fluid | A mixture of NOx and pure synthetic air |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhiltsova., T.; Martins, N.; Silva, M.R.F.; Silva, C.F.D.; Lourenço, M.A.O.; Tobaldi, D.M.; Covita, D.; Seabra, M.P.; Ferreira, P. Experimental and Computational Analysis of NOx Photocatalytic Abatement Using Carbon-Modified TiO2 Materials. Catalysts 2020, 10, 1366. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10121366
Zhiltsova. T, Martins N, Silva MRF, Silva CFD, Lourenço MAO, Tobaldi DM, Covita D, Seabra MP, Ferreira P. Experimental and Computational Analysis of NOx Photocatalytic Abatement Using Carbon-Modified TiO2 Materials. Catalysts. 2020; 10(12):1366. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10121366
Chicago/Turabian StyleZhiltsova., Tatiana, Nelson Martins, Mariana R. F. Silva, Carla F. Da Silva, Mirtha A. O. Lourenço, David M. Tobaldi, Daniel Covita, Maria Paula Seabra, and Paula Ferreira. 2020. "Experimental and Computational Analysis of NOx Photocatalytic Abatement Using Carbon-Modified TiO2 Materials" Catalysts 10, no. 12: 1366. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10121366