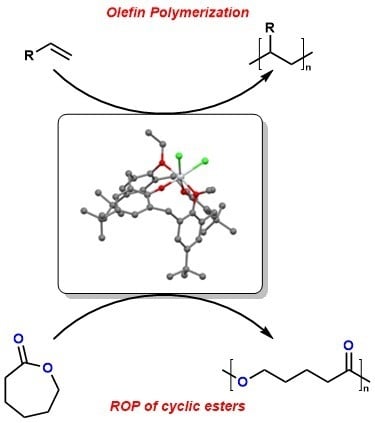
Use of Titanium Complexes Bearing Diphenolate or Calix[n]arene Ligands in α-Olefin Polymerization and the ROP of Cyclic Esters
Abstract
:1. Introduction
2. Olefin Polymerization
2.1. Titanium-Diphenolate Complexes
2.2. Titanocalix[n]arene Complexes
3. Ring Opening Polymerization (ROP)
3.1. Ti-diphenolate complexes
3.2. Titanocalix[n]arene complexes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Homden, D.H.; Redshaw, C. The Use of Calixarenes in Metal-Based Catalysis Chem. Review 2008, 108, 5086–5130. [Google Scholar]
- Li, Y.; Zhao, K.-Q.; Redshaw, C.; Martínez Ortega, B.A.; Nuñez, A.Y.; Hanna, T.A. Patai’s Chemistry of Functional Groups. Coordination Chemistry and Applications of Phenolic Calixarene–metal Complexes; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Amor, F.; Fokken, S.; Kleinhenn, T.; Spaniol, T.P.; Okuda, J. Mono(cyclopentadienyl)titanium complexes containing a sulfide-bridged bis(phenolato) ligand. Molecular structure of Ti{2,2′-S(OC6H2-4-Me-6-tBu)2}(η5-C5H5)Cl. J. Organomet. Chem. 2001, 621, 3–9. [Google Scholar] [CrossRef]
- Olmstead, M.M.; Sigel, G.; Hope, H.; Xu, X.; Power, P.P. Metallocalixarenes: Syntheses and X-Ray crystal structures of titanium(IV), iron(III), and cobalt(II) complexes of p-tert-butylcalix[4]arene. J. Am. Chem. Soc. 1985, 107, 8087–8091. [Google Scholar] [CrossRef]
- Kissin, Y.V.; Mink, R.I.; Brandolini, A.J.; Nowlin, T.E. AlR2Cl/MgR2 Combinations as Universal Cocatalysts for Ziegler–Natta, Metallocene, and Post-Metallocene Catalysts. J. Polym. Sci. Pol. Chem 2009, 47, 3271–3285. [Google Scholar] [CrossRef]
- Miyatake, T.; Mizunuma, K.; Seki, Y.; Kakugo, M. 2,2’-Thiobis(6-tert-butyl-4-methylphenoxy)titanium or zirconium complex-methylalumoxane catalysts for polymerization of olefins. Makromol. Chem., Rapid Commun. 1989, 10, 349–352. [Google Scholar] [CrossRef]
- van der Linden, A.; Schaverien, C.J.; Meijboon, N.; Canter, C.; Orpen, A.G. Polymerization of a-Olefins and Butadiene and Catalytic Cyclotrimerization of 1-Alkynes by a New Class of Group IV Catalysts. Control of Molecular Weight and Polymer Microstructure via Ligand Tuning in Sterically Hindered Chelating Phenoxide T.itanium and Zirconium Species. J. Am. Chem. Soc. 1995, 117, 3008–3021. [Google Scholar] [CrossRef]
- Nakayama, Y.; Watanabe, K.; Ueyama, N.; Nakamura, A.; Harada, A.; Okuda, J. Titanium Complexes Having Chelating Diaryloxo Ligands Bridged by Tellurium and Their Catalytic Behavior in the Polymerization of Ethylene. Organometallics 2000, 19, 2498–2503. [Google Scholar] [CrossRef]
- Froese, R.D.J.; Musaev, D.G.; Matsubara, T.; Morokuma, K. Theoretical Studies of Ethylene Polymerization Reactions Catalyzed by Zirconium and Titanium Chelating Alkoxide Complexes. J. Am. Chem. Soc. 1997, 119, 7190–7196. [Google Scholar] [CrossRef]
- Umare, P.S.; Rao, K.; Tembe, G.L.; Dhoble, D.A.; Trivedi, B. Controlled Synthesis of Low-Molecular-Weight Polyethylene Waxes by Titanium–Biphenolate–Ethylaluminum Sesquichloride Based Catalyst Systems. J. Appl. Polym. Sci. 2007, 104, 1531–1539. [Google Scholar] [CrossRef]
- Umare, P.S.; Rao, K.; Tembe, G.L.; Dhoble, D.A.; Trivedi, B. Polyethylene Waxes: Catalytic Synthesis by Ti-Biphenolates. J. Macromol. Sci. A. 2007, 44, 977–987. [Google Scholar] [CrossRef]
- Ozerova, O.V.; Rathb, N.P.; Ladipo, F.T. Synthesis, characterization, and reactivity of titanium(IV) complexes supported by proximally bridged p-tert-butylcalix[4]arene ligands J. Organomet. Chem. 1999, 586, 223–233. [Google Scholar] [CrossRef]
- Frediani, M.; Sémeril, D.; Comucci, A.; Bettucci, L.; Frediani, P.; Rosi, L.; Matt, D.; Toupet, L.; Kaminsky, W. Ultrahigh-Molecular-Weight Polyethylene by Using a Titanium Calix[4]arene Complex with High Thermal Stability under Polymerization Conditions. Macromol. Chem. Phys. 2007, 208, 938–945. [Google Scholar] [CrossRef] [Green Version]
- Capacchione, C.; Neri, P.; Proto, A. Polymerization of ethylene in the presence of 1,3-dimethoxy-p-But-calix[4]arene titanium dichloride. NMR evidence of the cationic titanium compound generated by methylalumoxane. Inorg. Chem. Commun. 2003, 6, 339–342. [Google Scholar] [CrossRef]
- Espinas, J.; Darbost, U.; Pelletier, J.; Jeanneau, E.; Duchamp, C.; Bayard, F.; Boyron, O.; Broyer, J.-P.; Thivolle-Cazat, J.; Basset, J.-M.; et al. Titanacalixarenes in Homogeneous Catalysis: Synthesis, Conformation and Catalytic Activity in Ethylene Polymerisation. Eur. J. Inorg. Chem. 2010, 1349–1359. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.S.; Xie, G.H. Synthesis of calixtitanium compounds and using for ethylene and styrene polymerizations. Acta Polym. Sin. 1998, 101–103. [Google Scholar]
- Chen, Y.; Zhang, Y.; Shen, Z.; Sun, W. Polymerization of ethylene with calix[4]titanium-Al(i-Bu)3 systems. Acta. Polym. Sin. 2000, 2, 239–241. [Google Scholar]
- Diaz-Barrios, A.; Liscano, J.; Trujillo, M.; Agrifoglio, G.; Matos, J.O. Assignee: Intevep S.A. U.S. Patent 5,767,034, 1998. [Google Scholar]
- Matos, J.O.; Diaz-Barrios, A.; Liscano, J.; Trujillo, M.; Agrifoglio, G. Assignee: Intevep S.A. European Patent EP1125951, 2001. [Google Scholar]
- Morohashi, N.; Hattori, T.; Yokomakura, K.; Kabutob, C.; Miyanoa, S. Dinuclear titanium(IV) complex of p-tert-butylthiacalix[4]arene as a novel bidentate Lewis acid catalyst. Tetrahedron Lett. 2002, 43, 7769–7772. [Google Scholar] [CrossRef]
- Proto, A.; Giugliano, F.; Capacchione, C. Ethylene polymerization promoted by dinuclear titanium p-tert-butylthiacalix[4]arene complexes. Eur. Polym. J. 2009, 45, 2138–2141. [Google Scholar] [CrossRef]
- Takeuchi, D.; Nakamura, T.; Aida, T. Bulky Titanium Bis(phenolate) Complexes as Novel Initiators for Living Anionic Polymerization of ε-Caprolactone. Macromolecules 2000, 33, 725–729. [Google Scholar] [CrossRef]
- Takeuchi, D.; Aida, T. Sequential Cationic and Anionic Polymerizations by Triflate Complexes of Bulky Titanium Bisphenolates: One-Pot Synthesis of Polyoxetane-Poly(E-caprolactone) Block Copolymer. Macromolecules 2000, 33, 4607–4609. [Google Scholar] [CrossRef]
- Takashima, Y.; Nakayama, Y.; Watanabe, H.; Itono, T.; Ueyama, N.; Nakamura, A.; Yasuda, H.; Harada, A.; Okuda, J. Polymerizations of Cyclic Esters Catalyzed by Titanium Complexes Having Chalcogen-Bridged Chelating Diaryloxo Ligands. Macromolecules 2002, 35, 7538–7544. [Google Scholar] [CrossRef]
- Takashima, Y.; Nakayama, Y.; Hirao, T.; Yasuda, H.; Harada, A. Bis(amido)titanium complexes having chelating diaryloxo ligands bridged by sulfur or methylene and their catalytic behaviors for ring-opening polymerization of cyclic esters. J. Organomet. Chem. 2004, 689, 612–619. [Google Scholar] [CrossRef]
- Ejfler, J.; Kobyłka, M.; Jerzykiewicz, L.B.; Sobota, P. Titanium complexes supported by bis(aryloxo) ligand: Structure and lactide polymerization activities. J. Mol. Catal. A: Chem. 2006, 257, 105–111. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Liu, M.-Y.; Sutar, A.K.; Lin, C.-C. Synthesis and Structural Studies of Heterobimetallic Alkoxide Complexes Supported by Bis(phenolate) Ligands: Efficient Catalysts for Ring-Opening Polymerization of L-Lactide. Inorg. Chem. 2010, 49, 665–674. [Google Scholar] [CrossRef]
- Jiang, M.-T.; Kosuru, S.R.; Lee, Y.H.; Lu, W.-Y.; Vandavasi, J.K.; Lai, Y.-C.; Chiang, M.Y.; Chen, H.-Y. Factors influencing catalytic behavior of titanium complexes bearing bisphenolate ligands toward ring-opening polymerization of L-lactide and ε-caprolactone. Express Polym. Lett. 2018, 12, 126–135. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, Y.; Santoro, O.; Elsegood, M.R.J.; Bedwell, E.V.; Zahra, K.; Walton, A.; Redshaw, C. Use of titanocalix[4]arenes in the ring opening polymerization of cyclic esters. Cat. Sci. & Tech 2020. in-press. [Google Scholar] [CrossRef]
- Frediani, M.; Sémeril, D.; Mariotti, A.; Rosi, L.; Frediani, P.; Rosi, L.; Matt, D.; Toupet, L. Ring Opening Polymerization of Lactide under Solvent-Free Conditions Catalyzed by a Chlorotitanium Calix[4]arene Complex. Macromol. Rapid Commun. 2008, 29, 1554–1560. [Google Scholar] [CrossRef]
- Frediani, M.; Sémeril, D.; Matt, D.; Rosi, L.; Frediani, P.; Rizzolo, F.; Papini, A.M. Ring-Opening Polymerisation of rac-Lactide Using a Calix[4]arene-Based Titanium (IV) Complex. Int. J. Polym. Sci. 2010, 6. [Google Scholar] [CrossRef] [Green Version]
- Walton, M.J.; Lancaster, S.J.; Redshaw, C. Highly selective and immortal magnesium calixarene complexes for the ring-opening polymerization of rac-lactide. ChemCatChem 2014, 6, 1892–1898. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Wang, W.-C.; Hsu, C.-P.; Lu, W.-Y.; Chuang, W.-J.; Chiang, M.Y.; Laia, Y.-C.; Chen, H.-Y. The ring-opening polymerization of ε-caprolactone and L-lactide using aluminum complexes bearing benzothiazole ligands as catalysts. Polym. Chem. 2016, 7, 4367–4377. [Google Scholar] [CrossRef]
- Florcza, M.; Duda, A. Effect of the configuration of the active center on comonomer reactivities: The case of epsilon-caprolactone/L,L-lactide copolymerization. Angew. Chem., Int. Ed. 2008, 47, 9088–9091. [Google Scholar] [CrossRef]
- Duda, A.; Biela, T.; Libiszowski, J.; Penczek, S.; Dubois, P.; Mecerreyes, D.; Jérôme, R. Block and random copolymers of є-caprolactone. Polym. Degrad. Stab. 1998, 59, 215–222. [Google Scholar] [CrossRef]
- Zhong, Z.; Dijkstra, P.J.; Feijen, J. Controlled ring-opening polymerization of ω-pentadecalactone with yttrium isopropoxide as an initiator. Macromol. Chem. Phys. 2000, 201, 1329–1333. [Google Scholar] [CrossRef]
- Lebedev, B.; Yevstropov, A. Thermodynamic properties of polylactones. Makromol. Chem. 1984, 185, 1235–1253. [Google Scholar] [CrossRef]
- Naumann, S.; Scholten, P.B.V.; Wilson, J.A.; Dove, A.P. Dual Catalysis for Selective Ring-Opening Polymerization of Lactones: Evolution toward Simplicity. J. Am. Chem. Soc. 2015, 137, 14439–14445. [Google Scholar] [CrossRef]
- Jedliński, Z.; Juzwa, M.; Adamus, G.; Kowalczuk, M.; Montaudo, M. Anionic polymerization of pentadecanolide. A new route to a potentially biodegradable aliphatic polyester. Macromol. Chem. Phys. 1996, 197, 2923–2929. [Google Scholar] [CrossRef]
- Bouyahyi, M.; Duchateau, R. Metal-Based Catalysts for Controlled Ring-Opening Polymerization of Macrolactones: High Molecular Weight and Well-Defined Copolymer Architectures. Macromolecules 2014, 47, 517–524. [Google Scholar] [CrossRef] [Green Version]
- Grosso-Giordano, N.A.; Solovyov, A.; Hwang, S.; Katz, A. Effect of Coordination Environment in Grafted Single-Site Ti-SiO2 Olefin Epoxidation Catalysis. Top Catal. 2016, 59, 1110–1122. [Google Scholar] [CrossRef]
- Ryan, J.D.; Gagnon, K.J.; Teat, S.J.; McIntosh, R.D. Flexible macrocycles as versatile supports for catalytically active metal clusters. Chem. Commun. 2016, 52, 9071–9073. [Google Scholar] [CrossRef] [Green Version]






















| Entry | Complex | Monomer | Activity a | Mw (10−4) | Mw/Mn |
|---|---|---|---|---|---|
| 1 | 1 | ethylene | 228 | 360 | 2.0 |
| 2 | 2 | ethylene | 188 | 420 | 2.5 |
| 3 | 1 | propylene | 186 | >800 | 2.2 |
| 4 | 2 | propylene | 87 | >800 | 2.0 |
| 5 | [Ti(OiPr)4] | propylene | 4.8 | 41.5 | 6.9 |
| Entry | Complex | Time (min) | Activity (kgPE × molTi−1 × h−1) | Mn (10−3) | Mw/Mn |
|---|---|---|---|---|---|
| 1 | 1 | 5 | 4740 | 29.5 | 11.9 |
| 2 | 3 | 15 | 504 | 50.0 | 7.0 |
| 3 | 4 | 10 | 390 | nd | nd |
| 4 | 5 | 15 | 180 | nd | nd |
| Entry | Complex | Time (h) | Activity (kgPE × molTi−1 × atm−1 × h−1) | Mn (10−4) | Mw/Mn |
|---|---|---|---|---|---|
| 1 | 6 | 0.08 | 161 | - | |
| 2 | 6 | 1 | 70 | 1.5 | 3.2 |
| 3 | 6 | 3 | 21 | - | - |
| 4 | 7 | 6 | 0.34 | 1.5 | 19 |
| 5 | 8 | 1 | 29 | 2.5 | 4.1 |
| 6 | 9 | 1 | 130 | - | - |
| 7 | 10 | 6 | 2.3 | 2.4 | 6.1 |
| 8 | 1 | 1 | 220 | 0.8 | 2.2 |
| 9 | 4 | 1 | 1.3 | - | - |
| Entry | Complex | Activity (kgPE × gTi−1) | Mw | Mw/Mn |
|---|---|---|---|---|
| 1 | 11 | 7.8 | 1900 | 1.7 |
| 2 | 12 | 3.5 | 770 | 1.3 |
| 3 | 13 | 11.0 | 1760 | 1.5 |
| 4 | 14 | 4.2 | 3380 | 1.9 |
| 5 | 15 | 9.5 | 1290 | 1.5 |
| 6 | Cp2TiCl2 | - | - | - |
| Entry | Solvent | [Al] | T (°C) | Pethylene (atm) | Activity (kgPE × gTi−1) | Mw | Mw/Mn | Tm (°C) |
|---|---|---|---|---|---|---|---|---|
| 1 | Toluene | EASC | 100 | 20 | 9.5 | 1290 | 1.5 | 118 |
| 2 | Toluene | Et2AlCl | 100 | 20 | 6.7 | - | - | 113 |
| 3 | Toluene | MAO | 100 | 20 | 2.1 | - | - | 136 |
| 4 | Toluene | EtAlCl2 | 100 | 20 | 1.8 | - | - | 125 |
| 5 | Toluene | Et3Al | 100 | 20 | 0.2 | - | - | - |
| 6 | Toluene | EASC | 30 | 20 | 0.4 | - | - | - |
| 7 | Toluene | EASC | 60 | 20 | 2.3 | - | - | 128 |
| 8 | Toluene | EASC | 100 | 7 | 3.5 | 890 | 1.3 | 111 |
| 9 | Toluene | EASC | 100 | 33 | 18.3 | 3200 | 1.6 | 119 |
| 10 | Hexane | EASC | 100 | 20 | 0.8 | - | - | - |
| 11 | Chlorobenzene | EASC | 100 | 20 | 12.4 | 1550 | 1.6 | 117 |
| Entry | Complex (mmol) | MAO (Al/Ti) | Time (min) | Activity (kg molcat−1 h−1) |
|---|---|---|---|---|
| 1 | 16 (0.02) | 500 | 30 | 9 |
| 2 | 17 (0.02) | 500 | 30 | 70 |
| 3 | 18 (0.02) | 500 | 30 | 15 |
| 4 | 19 (0.02) | 500 | 30 | 35 |
| 5 | 20 (0.02) | 500 | 30 | 15 |
| 6 | 21 (0.02) | 500 | 30 | 25 |
| 7 | 22 (0.02) | 500 | 30 | 24 |
| Entry | T (°C) | [ethylene] (M) | Activity (kgpolym. molcat−1 h−1) | Tm (°C) | Mηα (10−6) |
|---|---|---|---|---|---|
| 1 | 45 | 0.5 | 10.6 | 135.8 | 3.98 |
| 2 | 60 | 0.5 | 29.8 | 136.9 | 3.44 |
| 3 | 75 | 0.5 | 43.7 | 137.0 | 2.54 |
| 4 | 90 | 0.5 | 60.0 | 134.2 | 0.96 |
| 5 | 105 | 0.5 | 54.5 | 137.0 | 0.73 |
| 6 | 120 | 0.5 | 27.0 | 135.5 | 0.60 |
| 7 | 60 | 0.25 | 9.8 | 138.3 | 3.24 |
| 8 | 60 | 0.75 | 53.8 | 137.8 | 3.53 |
| 9 | 60 | 1.00 | 82.4 | 138.3 | 4.22 |
| Entry | Complex | Activity (kgpolym. molcat−1 h−1) | Mn (10−6) | Mw/Mn | Tm (°C) |
|---|---|---|---|---|---|
| 1 | 19 | 113 | 1.7 | 4.4 | 137.4 |
| 2 | 24 | 14 | 2.9 | 2.7 | 132.0 |
| 3 | 25 | 16 | 2.7 | 2.5 | 131.8 |
| 4 | 26 | 21 | 3.2 | 2.9 | 131.4 |
| 5 | 27 | 16 | 2.3 | 3.0 | 133.2 |
| 6 | 28 | 185 | 2.6 | 3.3 | 134.6 |
| 7 | 29 | 350 | 1.4 | 5.4 | 133.6 |
| 8 | 30 | 83 | n.d. a | n.d. a | 137.4 |
| Entry | Complex | Activity (gpolym molcat−1 h−1) | Mw (10−6) | Tm (°C) |
|---|---|---|---|---|
| 1 | 32 | 1.36 | 1.22 | 136.7 |
| 2 | 33 | 1.84 | 5.06 | 141.5 |
| 3 | 34 | 2.04 | 1.74 | 139.6 |
| Entry | Complex | Activity (gpolym. molcat−1 h−1) | S-PS (%) | Tm (°C) |
|---|---|---|---|---|
| 1 | 32 | 6.59 | 90.6 | 265.6 |
| 2 | 33 | 6.89 | 92.3 | 270.4 |
| 3 | 34 | 7.56 | - | - |
| Entry | AlR3 | Al/Ti | T (°C) | Time (h) | Activity (gpolym. molcat−1 h−1) | Tm (°C) |
|---|---|---|---|---|---|---|
| 1 | Al(iBu)3 | 10 | 35 | 1 | - | - |
| 3 | Al(iBu)3 | 15 | 35 | 1 | 31.5 | 137.9 |
| 3 | Al(iBu)3 | 20 | 35 | 1 | 60.7 | 137.8 |
| 4 | Al(iBu)3 | 40 | 35 | 1 | 66.2 | 136.1 |
| 5 | Al(iBu)3 | 20 | 0 | 1 | 28.7 | 136.7 |
| 6 | Al(iBu)3 | 20 | 0 | 4 | 15.7 | nd |
| 7 | Al(iBu)3 | 20 | 35 | 1 | 60.7 | 137.8 |
| 8 | Al(iBu)3 | 20 | 60 | 1 | 60.0 | 137.5 |
| 9 | Al(iBu)3 | 20 | 100 | 1 | 37.3 | 134.6 |
| 10 | Al(iBu)3 | 20 | 100 | 4 | 9.7 | nd |
| 11 | AlEt3 | 40 | 35 | 1 | 83.3 | nd |
| 12 | Al(iBu)3 | 40 | 35 | 1 | 66.2 | nd |
| 13 | AlEt2Cl | 40 | 35 | 1 | 30.0 | nd |
| 14 | Al(iBu)2Cl | 40 | 35 | 1 | 14.7 | nd |
| Entry | Complex | T (°C) | Time (h) | Pethylene (atm) | Activity b | Mn (10−4) | Mw/Mn | Tm (°C) |
|---|---|---|---|---|---|---|---|---|
| 1 | 36 | 0 | 2 | 1 | 19 | 1.9 | 29.3 c | 133.1 |
| 2 | 36 | 25 | 2 | 1 | 25 | 1.1 | 26.6 c | 132.8 |
| 3 | 36 | 50 | 2 | 1 | 4.7 | 1.1 | 41.3 c | 132.0 |
| 4 | 36 | 25 a | 1.5 | 5 | 15 | 30 | 2.6 | 134.1 |
| 5 | 36 | 25 a | 1 | 5 | 13 | 25 | 3.9 | 138.4 |
| 6 | 36 | 50 a | 1 | 5 | 11 | 4.6 | 15.8 c | 133.0 |
| 7 | 37 | 0 | 2 | 1 | 6.5 | 35 | 4.0 | 142.1 |
| 8 | 37 | 25 | 2 | 1 | 7.7 | 4.5 | 11.6 c | 136.8 |
| 9 | 37 | 50 | 2 | 1 | 4.3 | 1.5 | 41.2 c | 133.4 |
| 10 | 37 | 25 a | 1 | 5 | 14 | 1.2 | 54.7 c | 136.5 |
| 11 | 37 | 50 a | 1 | 5 | 12 | 42 | 13.2 c | 132.9 |
| Entry | Complex | Monomer | Solvent | T (°C) | Time (h) | Yield (%) | Mn (103) | Mw/Mn |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | ε-CL | none | 100 | 6 | 93 | 62.2 | 2.07 |
| 2 | 1 | ε-CL | toluene | 100 | 6 | 100 | 72.3 | 2.28 |
| 3 a | 1 | ε-CL | anisole | 100 | 6 | 94 | 28.1 | 1.17 |
| 4 | 6 | ε-CL | none | 100 | 12 | 55 | 45.1 | 1.65 |
| 5 | 6 | ε-CL | toluene | 50 | 6 | <1 | - | |
| 6 | 6 | ε-CL | toluene | 100 | 6 | 88 | 26.0 | 1.20 |
| 7 a | 6 | ε-CL | anisole | 100 | 6 | 87 | 11.0 | 1.09 |
| 8 a | 6 | ε-CL | dioxane | 100 | 6 | 90 | 17.6 | 1.06 |
| 9 | 8 | ε-CL | none | 100 | 12 | 85 | 37.9 | 1.43 |
| 10 | 9 | ε-CL | toluene | 100 | 6 | <1 | - | - |
| 11 | 10 | ε-CL | toluene | 100 | 6 | <1 | - | - |
| 12 | 6 | δ-VL | none | 100 | 6 | 51 | 38.4 | 1.52 |
| 13 | 6 | δ-VL | toluene | 100 | 6 | 49 | 24.5 | 1.29 |
| 14 | 8 | δ-VL | none | 100 | 6 | 82 | 62.4 | 1.64 |
| 15 | 8 | δ-VL | toluene | 100 | 6 | <1 | - | - |
| 16 | 6 | β-PL | toluene | 100 | 6 | <1 | - | - |
| 17 a | 6 | L-LA | anisole | 100 | 8 | 18 | 4.9 | 1.07 |
| 18 a | 6 | L-LA | anisole | 100 | 48 | 98 | 28.2 | 1.08 |
| Entry | Complex | Monomer | Time (h) | Yield (%) | Mn (103) | Mw/Mn |
|---|---|---|---|---|---|---|
| 1 | 47 | ε-CL | 4 | 56.9 | 8.2 | 1.09 |
| 2 | 47 | ε-CL | 32 | 90.3 | 18.8 | 1.31 |
| 3 | 48 | ε-CL | 2 | 23.5 | 15.3 | 1.16 |
| 4 | 48 | ε-CL | 8 | 91.8 | 56.2 | 1.60 |
| 5 | 47 | L-LA | 36 | 24.4 | 7.6 | 1.08 |
| 6 | 47 | L-LA | 120 | 90.3 | 20.4 | 1.23 |
| Entry | Complex | [M]0/[Ti]0 | Time (h) | Conversiona (%) | Mnb,c | Mw/Mn b |
|---|---|---|---|---|---|---|
| 1 | 48 | 100 | 1.5 | 76 | 5400 | 1.08 |
| 2 | 49 | 150 | 1.5 | 74 | 5300 | 1.07 |
| 3 | 50 | 150 | 1.5 | 80 | 6100 | 1.05 |
| 4 | 51 | 100 | 0.5 | 91 | 8800 | 1.27 |
| 5 | 52 | 100 | 0.5 | 94 | 7800 | 1.16 |
| 6 | 52 | 200 | 0.5 | 95 | 14,800 | 1.15 |
| 7 | 53 | 100 | 0.25 | 93 | 6800 | 1.29 |
| 8 | 54 | 100 | 3.5 | 89 | 6300 | 1.28 |
| 9 d | 54 | 100 | 1.5 | 94 | 7900 | 1.18 |
| 10 e | 55 | 100 | 1 | 85 | 8700 | 1.19 |
| 11 e | 55 | 200 | 1 | 91 | 16,200 | 1.12 |
| 12 e | 56 | 100 | 1 | 95 | 6900 | 1.29 |
| 13 | 57 | 100 | 0.5 | 94 | 3800 | 1.17 |
| Entry | Complex | Monomer | Time (h) | Conversion a (%) | Mnb,c | Mw/Mn b |
|---|---|---|---|---|---|---|
| 1 | 58 | ε-CL | 2.0 | >99 | 4800 | 1.18 |
| 2 | 59 | ε-CL | 2.5 | 91 | 4100 | 1.11 |
| 3 | 60 | ε-CL | 2.5 | 88 | 7100 | 1.50 |
| 4 | 61 | ε-CL | 3.0 | 90 | 7000 | 1.34 |
| 5 | 62 | ε-CL | 4.5 | 92 | 6200 | 1.47 |
| 6 | 58 | L-LA | 2.0 | 91 | 4600 | 1.06 |
| 7 | 59 | L-LA | 2.0 | 94 | 5000 | 1.07 |
| 8 | 60 | L-LA | 3.7 | 92 | 11,000 | 1.18 |
| 9 | 61 | L-LA | 5.0 | 91 | 8200 | 1.20 |
| 10 | 62 | L-LA | 5.0 | 85 | 5600 | 1.12 |
| Run | Monomer | M:Ti:BnOH | T (℃) | Conv. (%) a | Mn(corr) b,c | Mn(calc) d | Mw/Mn b |
|---|---|---|---|---|---|---|---|
| 1 | ε-CL | 500:1:2 | 80 | 53 | 6390 | 15,140 | 1.24 |
| 2 | δ−VL | 500:1:2 | 80 | 35 | 7490 | 8860 | 1.15 |
| 3 | r-LA | 500:1:2 | 130 | 80 | 8870 | 28,900 | 1.61 |
| Entry | LA:Ti | Activity (kgPLA × mol−1 × h−1) | Tm (°C) | Mn (10−3) | Mw/Mn |
|---|---|---|---|---|---|
| 1 | 196 | 9.4 | 160 | 11 | 1.4 |
| 2 | 434 | 20.6 | 162 | 12 | 1.3 |
| 3 | 864 | 36.5 | 161 | 15 | 1.2 |
| 4 | 1900 | 74.5 | 160 | 37 | 1.2 |
| 5 a | 1896 | 87.8 | 163 | 6 | 1.1 |
| 6 b | 2050 | 21.1 | 165 | 42 | 1.3 |
| 7 c | 463 | 20.0 | n.d. | 52 | 1.4 |
| Entry | LA:Ti | Activity (kgPLA·mol(Ti)−1·h−1) | Mn (10−3 g mol−1) | Mw/Mn | Tg (°C) |
|---|---|---|---|---|---|
| 1 | 200 | 9.2 | 15.0 | 1.2 | 53.0 |
| 2 | 500 | 21.0 | 14.9 | 1.3 | 52.1 |
| 3 | 1000 | 42.3 | 22.4 | 1.2 | 50.0 |
| 4 | 1994 | 65.3 | 36.9 | 1.2 | 41.7 |
| Entry | Time (min) | Conversion a (%) | Activity b | Mn (10-3) | Mw/Mn | Tg (°C) |
|---|---|---|---|---|---|---|
| 1 | 20 | 3 | 2.9 | - | - | - |
| 2 | 40 | 38 | 17.1 | - | - | - |
| 3 | 60 | 68 | 19.4 | 17.4 | 1.3 | 46.0 |
| 4 | 80 | 88 | 18.9 | 20.9 | 1.3 | 50.1 |
| Run | Cat. | Mon. | Mon.:Ti:BnOH | T (°C) | Conv. (%) a | Mn b,c | PDI b |
|---|---|---|---|---|---|---|---|
| 1 | 24 | ε-CL | 500:1:2 | 80 | 32 | 3650 | 1.10 |
| 2 | 26 | ε-CL | 500:1:2 | 80 | 33 | 650 | 1.10 |
| 3 | 65 | ε-CL | 500:1:2 | 80 | 68 | 3640 | 1.10 |
| 4 | 66 | ε-CL | 500:1:2 | 80 | >99 | 9260 | 1.48 |
| 5 | 66 | ε-CL | 500:1:1 | 80 | >99 | 8100 | 1.40 |
| 6 | 67 | ε-CL | 500:1:2 | 80 | >99 | 19,030 | 1.55 |
| 7 d | 24 | ε-CL | 500:1:2 | 130 | >99 | 3580 | 1.80 |
| 8 d | 26 | ε-CL | 500:1:2 | 130 | >99 | 3390 | 1.40 |
| 9 d | 65 | ε-CL | 500:1:2 | 130 | >99 | 1630 | 1.50 |
| 10 d | 66 | ε-CL | 500:1:2 | 130 | >99 | 4580 | 2.08 |
| 11 d | 67 | ε-CL | 500:1:2 | 130 | >99 | 11,770 | 2.47 |
| 12 | 24 | δ-VL | 500:1:2 | 80 | 50 | 5310 | 1.10 |
| 13 | 26 | δ-VL | 500:1:2 | 80 | 45 | 6090 | 1.13 |
| 14 | 65 | δ-VL | 500:1:2 | 80 | 44 | 5700 | 1.10 |
| 15 | 66 | δ-VL | 500:1:1 | 80 | 81 | 9550 | 1.50 |
| 16 | 67 | δ-VL | 500:1:2 | 80 | 78 | 18,830 | 1.60 |
| 17 d | 66 | δ-VL | 500:1:1 | 130 | 62 | 9480 | 1.45 |
| 18 | 24 | r-LA | 500:1:2 | 130 | 95 | 13,520 | 1.68 |
| 19 | 26 | r-LA | 500:1:2 | 130 | 97 | 11,170 | 1.94 |
| 20 | 65 | r-LA | 500:1:2 | 130 | 95 | 15,770 | 2.09 |
| 21 | 66 | r-LA | 500:1:1 | 130 | 97 | 28,830 | 1.94 |
| 22 | 67 | r-LA | 500:1:2 | 130 | 65 | 22,040 | 1.04 |
| 23 d | 66 | r-LA | 500:1:1 | 130 | 94 | 12,200 | 1.73 |
| Run | Monomer | Monomer:M:BnOH | T (℃) | t (h) | Conv. a (%) | Mn b,c | PDI b |
|---|---|---|---|---|---|---|---|
| 1 | ε-CL | 400:1:0 | 130 | 24 | >99 | 9750 | 2.09 |
| 2 | 400:1:1 | 130 | 24 | >99 | 4840 | 2.77 | |
| 3 | δ-VL | 400:1:0 | 130 | 24 | 61 | 23,260 | 2.48 |
| 4 | r-LA | 400:1:0 | 130 | 24 | 78 | 20,340 | 1.15 |
| 5 | ω-PDL | 400:1:0 | 130 | 24 | 2 | - | - |
| Run | Catalyst | Monomer | M:Ti:BnOH | T (°C) | Conv. (%) a | Mn b,c | PDI b |
|---|---|---|---|---|---|---|---|
| 1 | 63 | ε-CL | 500:1:2 | 80 | 53 | 6390 | 1.24 |
| 2 | 64 | 500:1:2 | 80 | 24 | 2540 | 1.13 | |
| 3 | 66 | 500:1:1 | 80 | >99 | 8100 | 1.40 | |
| 4 | 63 | δ-VL | 500:1:2 | 80 | 35 | 7490 | 1.15 |
| 5 | 64 | 500:1:2 | 80 | 33 | 5700 | 1.13 | |
| 6 | 66 | 500:1:1 | 80 | 81 | 12,570 | 1.37 | |
| 7 | 63 | r-LA | 500:1:2 | 130 | 80 | 8870 | 1.61 |
| 8 | 64 | 500:1:2 | 130 | 77 | 10,700 | 1.20 | |
| 9 | 66 | 500:1:1 | 130 | 97 | 28,830 | 1.94 | |
| 10 | 63 | ω-PDL | 500:1:2 | 130 | none | - | - |
| 11 | 64 | 500:1:2 | 130 | 12 | - | - | |
| 12 | 66 | 500:1:1 | 130 | 53 | 2835 d | nd |
| Complex | Co-catalyst | Conditions | Activity (kgPE/molTi.h.atm) | Mw (10−4) | Mw/Mn | Reference |
|---|---|---|---|---|---|---|
| 1 | MAO (1000 equiv.) | 25 °C, 1 h 1 atm | 220 | 0.8 | 2.2 | 8 |
| 2 | MAO (4700 equiv.) | 20 °C, 20 s, 1 atm | 120 | 420 | 2.5 | 6 |
| 4 | MAO (500 equiv.) | 20 °C, 15 min, 3 atm | 60 | nd | nd | 7 |
| 6 | MAO (1000 equiv.) | 25 °C, 1 h, 1 atm | 70 | 1.5 | 3.2 | 8 |
| 8 | 29 | 2.5 | 4.1 | |||
| 24 | MAO (500 equiv.) | 50 °C, 2 h, 30 atm | 0.47 | 290 | 2.7 | 15 |
| 28 | 6.2 | 260 | 3.3 | |||
| 29 | 11.7 | 140 | 5.4 | |||
| 32 | MAO (500 equiv.) | 40 °C, 30 min, 10 atm | 1.4 × 10−4 | 120 | nd | 16 |
| 33 | 1.8 × 10−4 | 500 | nd | |||
| 34 | 2.0 × 10−4 | 170 | nd | |||
| 36 | MAO (1000 equiv.) | 50 °C, 2 h, 1 atm | 4.7 | 1.1 | 41.3 | 21 |
| 37 | 4.3 | 1.5 | 41.2 |
| Complex | Monomer | Conditions | Conversion | Mn | Mw/Mn | Ref |
|---|---|---|---|---|---|---|
| 1 | ε-CL | Toluene, 100 °C, 6 h | 100 | 7230 | 2.28 | 22 |
| 6 | 88 | 2600 | 1.20 | |||
| 8 | Neat, 100 °C, 12 h | 85 | 3790 | 1.43 | ||
| 44 | Toluene, 100 °C, 32 h | 90 | 1880 | 1.31 | 25 | |
| 45 | Toluene, 100 °C, 8 h | 92 | 5620 | 1.60 | ||
| 6 | L-LA | Anisole, 100 °C, 48 h | 98 | 2820 | 1.08 | 22 |
| 49 | Toluene, 30 °C, 1.5 h | 74 | 5300 | 1.07 | 27 | |
| 50 | 80 | 6100 | 1.05 | |||
| 51 | Toluene, 30 °C, 0.5 h | 91 | 8800 | 1.27 | ||
| 54 | Toluene, 30 °C, 3.5 h | 89 | 6300 | 1.28 | ||
| 58 | Toluene, 50 °C, 2 h | 91 | 4600 | 1.06 | 28 | |
| 62 | Toluene, 50 °C, 5 h | 85 | 5600 | 1.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoro, O.; Redshaw, C. Use of Titanium Complexes Bearing Diphenolate or Calix[n]arene Ligands in α-Olefin Polymerization and the ROP of Cyclic Esters. Catalysts 2020, 10, 210. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10020210
Santoro O, Redshaw C. Use of Titanium Complexes Bearing Diphenolate or Calix[n]arene Ligands in α-Olefin Polymerization and the ROP of Cyclic Esters. Catalysts. 2020; 10(2):210. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10020210
Chicago/Turabian StyleSantoro, Orlando, and Carl Redshaw. 2020. "Use of Titanium Complexes Bearing Diphenolate or Calix[n]arene Ligands in α-Olefin Polymerization and the ROP of Cyclic Esters" Catalysts 10, no. 2: 210. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10020210