Co-Supported CeO2Nanoparticles for CO Catalytic Oxidation: Effects of Different Synthesis Methods on Catalytic Performance
Abstract
:1. Introduction
2. Results and Discussion
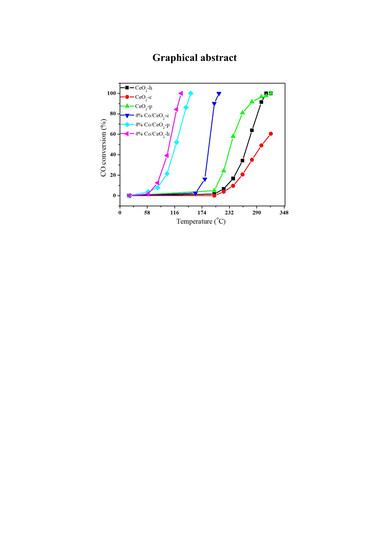
2.1. Catalytic Behavior
2.2. XRD and BET Analysis
2.3. SEM and TEM Analysis
2.4. Raman Spectroscopy
2.5. H2-TPR and O2-TPD Analysis
2.6. XPS Analysis
3. Materials and Methods
3.1. Synthesis of CeO2Nanoparticles
3.2. Synthesis of Co/CeO2Nanoparticles
3.3. Measurement of CO Oxidation Activity
3.4. Characterization of Materials
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guan-Hung, L.; Lak, J.H.; Tsai, D.-H. Hydrogen Production via Low-Temperature Steam–Methane Reforming Using Ni–CeO2–Al2O3 Hybrid Nanoparticle Clusters as Catalysts. ACS Appl. Energy Mater. 2019, 2, 7963–7971. [Google Scholar]
- Bruix, A.; Neyman, K. Modeling Ceria-Based nanomaterials for catalysis and related applications. Catal. Lett. 2016, 146, 2053–2080. [Google Scholar] [CrossRef]
- Alessandro, T.; Jordi, L. Ceria Catalysts at Nanoscale: How Do Crystal Shapes Shape Catalysis? ACSCatal. 2017, 7, 4716–4735. [Google Scholar]
- Divins, N.J.; Angurell, I.; Escudero, C.; Dieste, V.; Liorca, J. Nanomaterials Influence of the support on surface rearrangements of bimetallic nanoparticles in real catalysts. Science 2014, 346, 620–623. [Google Scholar] [CrossRef] [Green Version]
- Zhe, Z.; Jiafeng, Y.; Jixin, Z.; Qingjie, G.; Hengyong, X.; Felix, D.; Roland, D.; Jian, S. Tailored metastable Ce–Zr oxides with highly distorted lattice oxygen for accelerating redox cycles. Chem. Sci. 2018, 9, 3386–3394. [Google Scholar]
- Dvořák, F.; Farnesi, C.M.; Tovt, A.; Tran, N.D.; Negreiros, F.R.; Vorokhta, M. Creating single-atom Pt-ceria catalysts by surface step decoration. Nat. Commun. 2016, 7, 10801. [Google Scholar] [CrossRef]
- Jones, J.; Xiong, H.; Delariva, A.; Peterson, E.; Pham, H.; Challa, S.; Qi, G.; Oh, S.; Wiebenga, M.; Hernandez, X.; et al. Thermally stable single-atom platinum-on-ceriacatalysts via atomtrapping. Science 2016, 353, 150–154. [Google Scholar] [CrossRef] [Green Version]
- Devaiah, D.; Reddy, L.; Park, S.; Reddy, B. Ceria-zirconia mixed oxides: Synthetic methods and applications. Catal. Rev. 2018, 60, 177–277. [Google Scholar] [CrossRef]
- Lykaki, M.; Stefa, S.; Carabineiro, S.A.C.; Pandis, P.K.; Stathopoulos, V.N.; Konsolakis, M. Facet-Dependent Reactivity of Fe2O3/CeO2 Nanocomposites: Effect of CeriaMorphology on CO Oxidation. Catalysts 2019, 9, 371. [Google Scholar] [CrossRef] [Green Version]
- Lykaki, M.; Pachatouridou, E.; Carabineiro, S.A.C.; Iliopoulou, E.; Andriopoulou, C.; Kallithrakas-Kontos, N.; Boghosian, S.; Konsolakis, M. Ceria nanoparticles shape effffects on the structural defects and surface chemistry: Implications in CO oxidation by Cu/CeO2 catalysts. Appl.Catal. B 2018, 230, 8–28. [Google Scholar] [CrossRef]
- Kalasin, S.; Browne, E.P.; Arcaro, K.F.; Santore, M.M. Surfaces that Adhesively Discriminate Breast Epithelial Cell Lines and Lymphocytes in Buffffer and Human Breast Milk. ACS Appl. Mater. Interfaces 2019, 11, 17035–17049. [Google Scholar] [CrossRef] [PubMed]
- Puigdollers, A.R.; Pacchioni, G. CO Oxidation on Au Nanoparticles Supported on ZrO2: Role of Metal/Oxide Interface and Oxide Reducibility. ChemCatChem 2017, 9, 1119–1127. [Google Scholar] [CrossRef]
- Shen, W.; Mao, D.; Luo, Z.; Yu, J. CO oxidation on mesoporous SBA-15 supported CuO–CeO2 catalyst prepared by a surfactant-assisted impregnation method. RSC Adv. 2017, 7, 27689–27698. [Google Scholar] [CrossRef] [Green Version]
- Junhao, L.; Zhongqi, L.; David, A.C.; Wenhui, H.; Jier, H.; Libo, Y.; Zhenmeng, P.; Peilin, L.; Ruigang, W. Distribution and Valence State of Ru Species on CeO2 Supports: Support Shape Effect and Its Influence on CO Oxidation. ACS Catal. 2019, 9, 11088–11103. [Google Scholar]
- Dong, G.; Wang, J.; Gao, Y.; Chen, S. A Novel Catalyst for CO Oxidation at Low Temperature. Catal. Lett. 1999, 58, 37–41. [Google Scholar] [CrossRef]
- Spezzati, G.; Su, Y.; Hofmann, J.; Benavibez, A.; Delariva, A.; Mccabe, J.; Datye, A.; Hensen, E. Atomically dispersed Pd-O species on CeO2(111) as highly active sites for low-temperature CO oxidation. ACS Catal. 2017, 7, 6887–6891. [Google Scholar] [CrossRef] [Green Version]
- Gulyaev, R.; Slavinskaya, E.; Novopashin, S.; Smovzh, D.; Zaikovsdii, A.; Osadchii, D.; Bulavchenkoa, O.; Koreven, S.; Boronin, A. Highly active PdCeOx composite catalysts for low-temperature CO oxidation, prepared by plasma-arc synthesis. Appl. Catal. B 2014, 147, 132–143. [Google Scholar] [CrossRef]
- Yarxin, C.; Junxiao, C.; Weiye, Q.; George, C.; Aouine, M.; Vernous, P.; Xingfu, T. Well-Defined Palladium-Ceria Interfacial Electronic Effects Trigger CO Oxidation. Chem. Commun. 2018, 54, 10140–10143. [Google Scholar]
- Xin, J.; Yang, D.; Dapeng, L.; Xilan, F.; Wang, L.; Zheng, Z.; Yu, Z. CO Oxidation Catalyzed by Two-Dimensional Co3O4/CeO2 Nanosheets. ACS Appl. Nano Mater. 2019, 2, 5769–5778. [Google Scholar]
- Liang, J.; Yang, X.; Wang, A.; Zhang, T.; Li, J. Theoretical investigations of non-noble metal single-atom catalysis: Ni1/FeOx for CO oxidation. Catal. Sci. Technol. 2016, 6, 1017–1036. [Google Scholar] [CrossRef]
- Zhou, L.; Li, X.; Yao, Z.; Chen, Z.; Hong, M.; Zhu, R.; Liang, Y.; Zhao, J. Transition-Metal Doped Ceria Microspheres with Nanoporous Structures for CO Oxidation. Sci. Rep. 2016, 6, 23900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Wu, Z.; Peng, X.; Binder, A.; Chai, S.; Dai, S. Origin of active oxygen in a ternary CuOx/Co3O4-CeO2 catalyst for CO oxidation. J. Phys. Chem. C 2014, 118, 27870–27877. [Google Scholar] [CrossRef]
- Thomas, K.; Manos, M. Transition Metal Atoms Embedded in Graphene: How Nitrogen Doping Increases CO Oxidation Activity. ACS Catal. 2019, 9, 6864–6868. [Google Scholar]
- Cui, L.; Di, Z.; Yang, Y.; Wang, Y.; Zhang, X. Synthesis of highly efficient α-Fe2O3 catalysts for CO oxidation derived from MIL-100(Fe). J. Solid State Chem. 2017, 247, 168–172. [Google Scholar] [CrossRef]
- Kaplin, I.; Lokteva, S.; Golubina, E.; Maslakov, K.; Strokova, N.; Chernyak, S.; Lunin, V. Sawdust as an effective biotemplate for the synthesis of Ce0.8Zr0.2O2 and CuO–Ce0.8Zr0.2O2 catalysts for total COE oxidation. RSC Adv. 2017, 7, 51359–51372. [Google Scholar] [CrossRef] [Green Version]
- Jampaiah, D.; Venkataswamy, P.; Coyle, V.E.; Reddy, B.M.; Bhargava, S.K. Low-temperature CO oxidation over manganese, cobalt, and nickel doped CeO2 nanorods. RSC Adv. 2016, 6, 80541–80548. [Google Scholar] [CrossRef]
- Lee, J.; An, K. Catalytic CO Oxidation on Nanocatalysts. Top. Catal. 2018, 61, 986–1001. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Q.; Wang, Y.; Song, G.; Li, C.; Zhao, W.; Fan, W. Solubility Product Difference-Guided Synthesis of Co3O4-CeO2 Core-Shell Catalysts for CO Oxidation. Catal. Sci. Technol. 2016, 6, 7273–7279. [Google Scholar] [CrossRef]
- Narayana, B.; Mukri, B.; Ghosal, P.; Subrahmanyam, C. Mn Ion substituted CeO2 Nano spheres for Low Temperature CO Oxidation: The Promoting Effect of Mn Ions. Chem. Sel. 2016, 1, 3150–3158. [Google Scholar]
- Gao, Y.; Wang, W.; Chang, S.; Huang, W. Morphology Effect of CeO2 Support in the Preparatport Interaction, and Catalytic Performance of Pt/CeO2 Catalysts. ChemCatChem 2013, 5, 3610–3620. [Google Scholar] [CrossRef]
- Junemin, B.; Dongjae, S.; Hojin, J.; Beom-Sik, K.; Jeong Woo, H.; Hyunjoo, L. Highly Water-Resistant La-Doped Co3O4 Catalyst for CO Oxidation. ACS Catal. 2019, 9, 10093–10100. [Google Scholar]
- Lei, L.; Qilei, Y.; Changyu, Z.; Jinlong, Y.; Yue, P.; Junhua, L. Hollow-Structural Ag/Co3O4Nanocatalyst for CO Oxidation: Interfacial Synergistic Effect. ACS Appl. Nano Mater. 2019, 2, 3480–3489. [Google Scholar] [CrossRef]
- Rashad, M.; Rüsing, M.; Berth, G.; Lischka, K.; Pawlis, A. CuO and Co3O4 Nanoparticles: Synthesis, Characterizations, and Raman Spectroscopy. J.Nanomater. 2013, 2013, 714853. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Qin, X. Effect of cobalt oxide on surface structure of alumina supported molybdena catalysts studied by in situ Ramanspectroscopy. Catal. Lett. 1993, 18, 409–418. [Google Scholar] [CrossRef]
- Singhania, A.; Gupta, S. CeO2-xNx Solid Solutions: Synthesis, Characterization, Electronic Structure and Catalytic Study for CO Oxidation. Catal. Lett. 2018, 148, 2001–2007. [Google Scholar] [CrossRef]
- Niu, G.; Hildebrandt, E.; Schubert, M.; Boscherini, F.; Zoellner, M.; Alff, L.; Walczyk, D.; Zaumseil, P.; Costina, L.; Wilkens, H.; et al. Oxygen Vacancy Induced Room Temperature Ferromagnetism in Pr-Doped CeO2 Thin Films on Silicon. ACS Appl. Mater. Interfaces 2014, 6, 17496–17505. [Google Scholar] [CrossRef]
- LEE, Y.; He, G.; Akey, A.J.; Si, R.; Stephanopuulos, M. Raman analysis of mode softening in nanoparticle CeO(2-δ) and Au-CeO(2-δ) during CO oxidation. J. Am. Chem. Soc. 2011, 133, 12952–12955. [Google Scholar] [CrossRef]
- Parvulescu, V.; Tiseanu, C. Local structure in CeO2 and CeO2-ZrO2 nanoparticles probed by Eu luminescence. Catal. Today 2015, 253, 33–39. [Google Scholar] [CrossRef]
- Luo, J.; Meng, M.; Yao, J.; Li, J.; Zha, Y.; Wang, X.; Zhang, T. One-step synthesis of nanostructured Pd-doped mixed oxides MOx-CeO2 (M=Mn, Fe, Co, Ni, Cu) for efficient CO and C3H8 total oxidation. Appl.Catal. B 2009, 87, 92–103. [Google Scholar] [CrossRef]
- Yafeng, C.; Jia, X.; Yun, G.; Jingyue, L. Ultrathin, Polycrystalline, Two-Dimensional Co3O4 for Low-Temperature CO Oxidation. ACS Catal. 2019, 9, 2558–2567. [Google Scholar]
- Cronauer, D.; Kropf, A.; Marshall, C.; Gao, P.; Hopps, S.; Jacobs, G.; Davis, B. Fischer–Tropsch Synthesis: Preconditioning Effects Upon Co-Containing Promoted and Unpromoted Catalysts. Catal. Lett. 2012, 142, 698–713. [Google Scholar] [CrossRef]
- Reddy, B.; Rao, K.; Bharali, P. Copper Promoted Cobalt and Nickel Catalysts Supported on Ceria−Alumina Mixed Oxide: Structural Characterization and CO Oxidation Activity. Ind. Eng. Chem. Res. 2009, 48, 8478–8486. [Google Scholar] [CrossRef]
- Hou, X.; Wang, Y.; Zhao, Y. Effect of CeO2 Doping on Structure and Catalytic Performance of Co3O4 Catalyst for Low-Temperature CO Oxidation. Catal. Lett. 2008, 123, 321–326. [Google Scholar] [CrossRef]
- Qin, H.; Qian, X.; Tao, M.; Lin, Y.; Ma, Z. Pt/MOx/SiO2, Pt/MOx/TiO2, and Pt/MOx/Al2O3 Catalysts for CO Oxidation. Catal. 2015, 5, 606–633. [Google Scholar] [CrossRef]
- Jie, L.; Lu, G.; Wu, G.; Mao, D.; Wang, Y.; Guo, Y. Promotional role of ceria on cobaltosic oxide catalyst for low-temperature CO oxidation. Catal. Sci. Technol. 2012, 2, 1865–1871. [Google Scholar]
- Kaidong, W.; Can, W.; Feng, W.; Nan, J.; Guoqiang, J. Co/Co3O4 Nanoparticles Coupled with Hollow Nanoporous Carbon Polyhedrons for the Enhanced Electrochemical Sensing of Acetaminophen. ACS Sustain. Chem. Eng. 2019, 7, 18582–18592. [Google Scholar]
- Lou, Y.; Cao, X.; Lan, J.; Wang, L.; Dai, Q.; Guo, Y.; Ma, J.; Zhao, Z.; Guo, Y.; Hu, P.; et al. Ultralow-temperature CO oxidation on an In2O3-Co3O4catalyst: A strategy to tune CO adsorption strength and oxygen activation simultaneously. Chem.Commun. 2014, 50, 6835–6838. [Google Scholar] [CrossRef]
- Kezhi, L.; Jianjun, C.; Yue, P.; Weichen, L.; Tao, Y.; Junhua, L. The relationship between surface open cells ofa-MnO2 and CO oxidation ability from a surface point of view. J. Mater. Chem. A 2017, 5, 20911–20921. [Google Scholar]
- Hyunwoo, H.; Sinmyung, Y.; Kwangjin, A.; Hyun, Y.K. Catalytic CO Oxidation over Au Nanoparticles Supported on CeO2 Nanocrystals: Effect of the Au–CeO2 Interface. ACS Catal. 2018, 8, 11491–11501. [Google Scholar]
- Shan, W.; Liu, F.; He, H.; Shi, X.; Zhang, C. A superior Ce-W-Ti mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B 2012, 115, 100–106. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, C.; Li, J. Structure–activity relationship of VOx/CeO2 nanorod for NO removal with ammonia. Appl. Catal. B 2014, 144, 538–546. [Google Scholar] [CrossRef]
- Li, J.; Lu, G.; Wu, G.; Mao, D.; Guo, Y.; Wang, Y.; Guo, Y. Effect of TiO2 crystal structure on the catalytic performance of Co3O4/TiO2 catalyst forlow-temperature CO oxidation. Catal. Sci. Technol. 2014, 4, 1268–1275. [Google Scholar] [CrossRef]
- Chuanyi, J.; Xijun, W.; Wenhui, Z.; Zhunzhun, W.; Oleg, V.P.; Yi, L.; Jun, J. Catalytic Chemistry Predicted by a Charge Polarization Descriptor: Synergistic O2 Activation and CO Oxidation by Au–Cu Bimetallic Clusters on TiO2(101). ACS Appl. Mater. Interfaces 2019, 11, 9629–9640. [Google Scholar]
- Li, H.; Wu, C.; Li, Y.; Zhang, J. Superior activity of MnOx-CeO2/TiO2 catalyst for catalytic oxidation of elemental mercury at low flue gas temperatures. Appl. Catal. B 2012, 111, 381–388. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Zhao, Q.; Ke, J.; Xiao, H.; Lv, X.; Liu, S.; Tade, M.; Wang, S. Mwchanistic investigation of the enhanced NH3-SCR on cobalt-decorated Ce-Ti mixed oxide: In situ FTIR analysis for structure-activity aorrelation. Appl. Catal. B 2017, 300, 297–308. [Google Scholar] [CrossRef]











| Catalysts | Particle Size a(nm) | BET Surface Area (m2/g) | O2 Desorption b (mmol/g) | H2Consumption c (mmol/g) | H/Htheoretical d |
|---|---|---|---|---|---|
| CeO2-h | 10.0 | 128.1 | 0.82 | 1.19 | 0.59 |
| CeO2-p | 14.6 | 65.1 | 0.50 | 0.88 | 0.57 |
| CeO2-c | >100 | 40.6 | 0 | 0.07 | 0.24 |
| 4%Co/CeO2-h | 8.9 | 96.4 | 1.08 | 2.01 | 0.92 |
| 4%Co/CeO2-p | 12.5 | 56.1 | 0.94 | 1.58 | 0.91 |
| 4%Co/CeO2-c | >100 | 43.8 | 0 | 1.11 | 0.74 |
| CeO2-c | CeO2-p | CeO2-h | Co/CeO2-c | Co/CeO2-p | Co/CeO2-h | |
|---|---|---|---|---|---|---|
| D/F2g (%) | 77.96 | 82.46 | 97.42 | 74.68 | 78.9 | 80.74 |
| Catalysts | Atomic Ratio (%) | |
|---|---|---|
| Ce3+/Ce | Co3+/Co | |
| 4% Co/CeO2-h | 16.58 | 52.84 |
| 4% Co/CeO2-p | 13.50 | 48.53 |
| 4% Co/CeO2-c | 11.89 | 45.72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, C.; Xing, L.; Cai, X.; Wang, Y.; Zhou, Q.; Li, M. Co-Supported CeO2Nanoparticles for CO Catalytic Oxidation: Effects of Different Synthesis Methods on Catalytic Performance. Catalysts 2020, 10, 243. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10020243
Sui C, Xing L, Cai X, Wang Y, Zhou Q, Li M. Co-Supported CeO2Nanoparticles for CO Catalytic Oxidation: Effects of Different Synthesis Methods on Catalytic Performance. Catalysts. 2020; 10(2):243. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10020243
Chicago/Turabian StyleSui, Chao, LeHong Xing, Xue Cai, Yang Wang, Qi Zhou, and Minghao Li. 2020. "Co-Supported CeO2Nanoparticles for CO Catalytic Oxidation: Effects of Different Synthesis Methods on Catalytic Performance" Catalysts 10, no. 2: 243. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10020243