CeO2 for Water Remediation: Comparison of Various Advanced Oxidation Processes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterizations of Bare CeO2 and Grey (Modified) CeO2
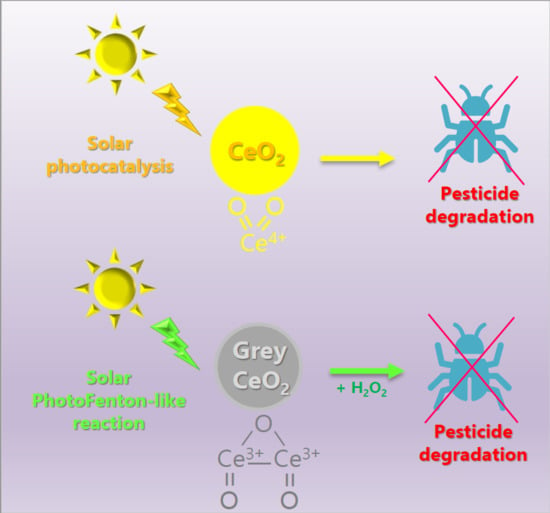
2.2. (Photo)catalytic Activity
2.3. Tocixity Tests
3. Materials and Methods
3.1. Samples Preparation
3.2. Samples Characterization
3.3. (Photo)catalytic Experiments
3.4. Toxicity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Salimi, M.; Esrafili, A.; Gholami, M.; Jonidi Jafari, A.; Rezaei Kalantary, R.; Farzadkia, M.; Kermani, M.; Sobhi, H.R. Contaminants of emerging concern: A review of new approach in AOP technologies. Environ. Monit. Assess. 2017, 189, 414. [Google Scholar] [CrossRef] [PubMed]
- Katsikantami, I.; Colosio, C.; Alegakis, A.; Tzatzarakis, M.N.; Vakonaki, E.; Rizos, A.K.; Sarigiannis, D.A.; Tsatsakis, A.M. Estimation of daily intake and risk assessment of organophosphorus pesticides based on biomonitoring data—The internal exposure approach. Food Chem. Toxicol. 2019, 123, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Malakootian, M.; Shahesmaeili, A.; Faraji, M.; Amiri, H.; Silva Martinez, S. Advanced oxidation processes for the removal of organophosphorus pesticides in aqueous matrices: A systematic review and meta-analysis. Process Saf. Environ. Prot. 2020, 134, 292–307. [Google Scholar] [CrossRef]
- Mazivila, S.J.; Ricardo, I.A.; Leitão, J.M.M.; Esteves da Silva, J.C.G. A review on advanced oxidation processes: From classical to new perspectives coupled to two- and multi-way calibration strategies to monitor degradation of contaminants in environmental samples. Trends Environ. Anal. Chem. 2019, 24, e00072. [Google Scholar] [CrossRef]
- Dewil, R.; Mantzavinos, D.; Poulios, I.; Rodrigo, M.A. New perspectives for Advanced Oxidation Processes. J. Environ. Manag. 2017, 195, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giménez, J.; Bayarri, B.; González, Ó.; Malato, S.; Peral, J.; Esplugas, S. Advanced Oxidation Processes at Laboratory Scale: Environmental and Economic Impacts. ACS Sustain. Chem. Eng. 2015, 3, 3188–3196. [Google Scholar] [CrossRef]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Iaria, C.; Scalisi, E.M.; Brundo, M.V.; Gulino, A.; Spitaleri, L.; Nicotra, G.; Dattilo, S.; et al. Preferential removal of pesticides from water by molecular imprinting on TiO2 photocatalysts. Chem. Eng. J. 2020, 379, 122309. [Google Scholar] [CrossRef]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Privitera, V.; Impellizzeri, G. Selective photodegradation of 2,4-D pesticide from water by molecularly imprinted TiO2. J. Photochem. Photobiol. A Chem. 2019, 380. [Google Scholar] [CrossRef]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Gulino, A.; Spitaleri, L.; Privitera, V.; Impellizzeri, G. Molecularly imprinted N-doped TiO2 photocatalysts for the selective degradation of o-phenylphenol fungicide from water. Mater. Sci. Semicond. Process. 2020, 112, 105019. [Google Scholar] [CrossRef]
- Cantarella, M.; Di Mauro, A.; Gulino, A.; Spitaleri, L.; Nicotra, G.; Privitera, V.; Impellizzeri, G. Selective photodegradation of paracetamol by molecularly imprinted ZnO nanonuts. Appl. Catal. B Environ. 2018, 238, 509–517. [Google Scholar] [CrossRef]
- Iervolino, G.; Zammit, I.; Vaiano, V.; Rizzo, L. Limitations and Prospects for Wastewater Treatment by UV and Visible-Light-Active Heterogeneous Photocatalysis: A Critical Review. Top. Curr. Chem. 2020, 378. [Google Scholar] [CrossRef] [PubMed]
- Herney-Ramirez, J.; Vicente, M.A.; Madeira, L.M. Heterogeneous photo-Fenton oxidation with pillared clay-based catalysts for wastewater treatment: A review. Appl. Catal. B Environ. 2010, 98, 10–26. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Zhang, J.; Luo, L.; Yang, Y.; Huang, H.; Peng, H.; Tang, L.; Mu, Y. Insight into electro-Fenton and photo-Fenton for the degradation of antibiotics: Mechanism study and research gaps. Chem. Eng. J. 2018, 347, 379–397. [Google Scholar] [CrossRef]
- Dutta, A.; Das, N.; Sarkar, D.; Chakrabarti, S. Development and characterization of a continuous solar-collector-reactor for wastewater treatment by photo-Fenton process. Sol. Energy 2019, 177, 364–373. [Google Scholar] [CrossRef]
- De la Obra, I.; Ponce-Robles, L.; Miralles-Cuevas, S.; Oller, I.; Malato, S.; Sánchez Pérez, J.A. Microcontaminant removal in secondary effluents by solar photo-Fenton at circumneutral pH in raceway pond reactors. Catal. Today 2017, 287, 10–14. [Google Scholar] [CrossRef]
- Sciré, S.; Fiorenza, R.; Gulino, A.; Cristaldi, A.; Riccobene, P.M. Selective oxidation of CO in H2-rich stream over ZSM5 zeolites supported Ru catalysts: An investigation on the role of the support and the Ru particle size. Appl. Catal. A Gen. 2016, 520, 82–91. [Google Scholar] [CrossRef]
- Fiorenza, R.; Spitaleri, L.; Gulino, A.; Sciré, S. High-Performing Au-Ag Bimetallic Catalysts Supported on Macro-Mesoporous CeO2 for Preferential Oxidation of CO in H2-Rich Gases. Catalysts 2020, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Fiorenza, R.; Condorelli, M.; D’Urso, L.; Compagnini, G.; Bellardita, M.; Palmisano, L.; Sciré, S. Catalytic and Photothermo-catalytic Applications of TiO2-CoOx Composites. J. Photocatal. 2020, 1. [Google Scholar] [CrossRef]
- Scuderi, V.; Amiard, G.; Sanz, R.; Boninelli, S.; Impellizzeri, G.; Privitera, V. TiO2 coated CuO nanowire array: Ultrathin p–n heterojunction to modulate cationic/anionic dye photo-degradation in water. Appl. Surf. Sci. 2017, 416, 885–890. [Google Scholar] [CrossRef]
- Di Mauro, A.; Fragalà, M.E.; Privitera, V.; Impellizzeri, G. ZnO for application in photocatalysis: From thin films to nanostructures. Mater. Sci. Semicond. Process. 2017, 69, 44–51. [Google Scholar] [CrossRef]
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; el Aatik, A.; Garrido, I.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Removal of Pesticides with Endocrine Disruptor Activity in Wastewater Effluent by Solar Heterogeneous Photocatalysis Using ZnO/Na2S2O8. Water Air Soil Pollut. 2019, 230, 134. [Google Scholar] [CrossRef]
- Bellardita, M.; Fiorenza, R.; Palmisano, L.; Sciré, S. Photocatalytic and photo-thermo-catalytic applications of cerium oxide based material. In Cerium Oxide (CeO2): Synthesis, Properties and Applications; a Volume in Metal Oxides Series; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-815661-2. [Google Scholar] [CrossRef]
- Heckert, E.G.; Seal, S.; Self, W.T. Fenton-like reaction catalyzed by the rare earth inner transition metal cerium. Environ. Sci. Technol. 2008, 42, 5014–5019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Issa Hamoud, H.; Azambre, B.; Finqueneisel, G. Reactivity of ceria–zirconia catalysts for the catalytic wet peroxidative oxidation of azo dyes: Reactivity and quantification of surface Ce(IV)-peroxo species. J. Chem. Technol. Biotechnol. 2016, 91, 2462–2473. [Google Scholar] [CrossRef]
- Zhang, N.; Tsang, E.P.; Chen, J.; Fang, Z.; Zhao, D. Critical role of oxygen vacancies in heterogeneous Fenton oxidation over ceria-based catalysts. J. Colloid Interface Sci. 2020, 558, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Hayat, W.; Zhang, Y.; Hussain, I.; Huang, S.; Du, X. Comparison of radical and non-radical activated persulfate systems for the degradation of imidacloprid in water. Ecotoxicol. Environ. Saf. 2020, 188, 109891. [Google Scholar] [CrossRef] [PubMed]
- Wamhoff, H.; Schneider, V. Photodegradation of imidacloprid. J. Agric. Food Chem. 1999, 47, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Tišler, T.; Jemec, A.; Mozetič, B.; Trebše, P. Hazard identification of imidacloprid to aquatic environment. Chemosphere 2009, 76, 907–914. [Google Scholar] [CrossRef]
- Zang, C.; Yu, K.; Hu, S.; Chen, F. Adsorption-depended Fenton-like reaction kinetics in CeO2-H2O2 system for salicylic acid degradation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 456–463. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J. Magnetic nanoscaled Fe3O4/CeO2 composite as an efficient fenton-like heterogeneous catalyst for degradation of 4-chlorophenol. Environ. Sci. Technol. 2012, 46, 10145–10153. [Google Scholar] [CrossRef]
- Aslam, M.; Qamar, M.T.; Soomro, M.T.; Ismail, I.M.I.; Salah, N.; Almeelbi, T.; Gondal, M.A.; Hameed, A. The effect of sunlight induced surface defects on the photocatalytic activity of nanosized CeO2 for the degradation of phenol and its derivatives. Appl. Catal. B Environ. 2016, 180, 391–402. [Google Scholar] [CrossRef]
- Fiorenza, R.; Spitaleri, L.; Gulino, A.; Scirè, S. Ru–Pd bimetallic catalysts supported on CeO2-MnOx oxides as efficient systems for H2 purification through CO preferential Oxidation. Catalysts 2018, 8, 203. [Google Scholar] [CrossRef] [Green Version]
- Fiorenza, R.; Bellardita, M.; Barakat, T.; Scirè, S.; Palmisano, L. Visible light photocatalytic activity of macro-mesoporous TiO2-CeO2 inverse opals. J. Photochem. Photobiol. A Chem. 2018, 352, 25–34. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Ce3+ and oxygen vacancy mediated tuning of structural and optical properties of CeO2 nanoparticles. Mater. Chem. Phys. 2012, 131, 666–671. [Google Scholar] [CrossRef]
- Ma, L.; Wang, D.; Li, J.; Bai, B.; Fu, L.; Li, Y. Ag/CeO2 nanospheres: Efficient catalysts for formaldehyde oxidation. Appl. Catal. B Environ. 2014, 148–149, 36–43. [Google Scholar] [CrossRef]
- Popovic, Z.V.; Dohcevic-Mitrovic, Z.; Konstantinovic, M.J.; Scepanovic, M. Raman scattering characterization of nanopowders and nanowires (rods). J. Raman Spectrosc. 2007, 38, 750–755. [Google Scholar] [CrossRef]
- Natile, M.M.; Boccaletti, G.; Glisenti, A. Properties and reactivity of nanostructured CeO2 powders: Comparison among two synthesis procedures. Chem. Mater. 2005, 17, 6272–6286. [Google Scholar] [CrossRef]
- Davydov, A. Molecular Spectroscopy of Oxide Catalyst Surfaces; Sheppard, N.T., Ed.; JohnWiley & Sons Ltd.: Chichester, UK, 2003. [Google Scholar]
- Liu, B.; Li, C.; Zhang, Y.; Liu, Y.; Hu, W.; Wang, Q.; Han, L.; Zhang, J. Investigation of catalytic mechanism of formaldehyde oxidation over three-dimensionally ordered macroporous Au/CeO2 catalyst. Appl. Catal. B Environ. 2012, 111, 467–475. [Google Scholar] [CrossRef]
- Finos, G.; Collins, S.; Blanco, G.; Del Rio, E.; Cíes, J.M.; Bernal, S.; Bonivardi, A. Infrared spectroscopic study of carbon dioxide adsorption on the surface of cerium-gallium mixed oxides. Catal. Today 2012, 180, 9–18. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everet, D.H.; Haul, R.A.W. Reporting Physisorption Data for gas/solid system with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Verma, R.; Samdarsh, S.K.; Bojja, S.; Paul, S.; Choudhury, B. A novel thermophotocatalyst of mixed-phase cerium oxide (CeO2/Ce2O3) homocomposite nanostructure: Role of interface and oxygen vacancies. Sol. Energy Mater. Sol. Cells 2015, 141, 414–422. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, W.; Zhang, L.; Zheng, Y.; Wang, Z. Insights into the Surface-Defect Dependence of Photoreactivity over CeO2 Nanocrystals with Well-Defined Crystal Facets. ACS Catal. 2015, 5, 4851–4858. [Google Scholar] [CrossRef]
- Mena, E.; Rey, A.; Rodríguez, E.M.; Beltrán, F.J. Nanostructured CeO2 as catalysts for different AOPs based in theapplication of ozone and simulated solar radiation. Catal. Today 2017, 280, 74–79. [Google Scholar] [CrossRef]
- Kim, Y.I.; Atherton, S.J.; Brigham, E.S.; Mallouk, T.E. Sensitized layered metal oxide semiconductor particles for photochemical hydrogen evolution from nonsacrificial electron donors. J. Phys. Chem. 1993, 97, 11802–11810. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, Y.; Zhong, Y.; Luo, Z.; Cen, K. The activity and characterization of CeO2 -TiO2 catalysts prepared by the sol—Gel method for selective catalytic reduction of NO with NH3. J. Hazard. Mater. 2010, 174, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Rosales, N.K.; Ayastuy, J.L.; González-Marcos, M.P.; Gutiérrez-Ortiz, M.A. Oxygen-enhanced water gas shift over ceria-supported Au-Cu bimetallic catalysts prepared by wet impregnation and deposition-precipitation. Int. J. Hydrogen Energy 2012, 37, 7005–7016. [Google Scholar] [CrossRef]
- Fiorenza, R.; Crisafulli, C.; Condorelli, G.G.; Lupo, F.; Scirè, S. Au-Ag/CeO2 and Au-Cu/CeO2 Catalysts for Volatile Organic Compounds Oxidation and CO Preferential Oxidation. Catal. Lett. 2015, 145, 1691–1702. [Google Scholar] [CrossRef]
- Bêche, E.; Charvin, P.; Perarnau, D.; Abanades, S.; Flamant, G. Ce 3d XPS investigation of cerium oxides and mixed cerium oxide (Ce xTiyOz). Surf. Interface Anal. 2008, 40, 264–267. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Brown, R.; Hashib, M.A. Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar] [CrossRef] [Green Version]
- Farré, M.J.; Franch, M.I.; Malato, S.; Ayllón, J.A.; Peral, J.; Doménech, X. Degradation of some biorecalcitrant pesticides by homogeneous and heterogeneous photocatalytic ozonation. Chemosphere 2005, 58, 1127–1133. [Google Scholar] [CrossRef]
- Verma, A.; Toor, A.P.; Prakash, N.T.; Bansal, P.; Sangal, V.K. Stability and durability studies of TiO2 coated immobilized system for the degradation of imidacloprid. New J. Chem. 2017, 41, 6296–6304. [Google Scholar] [CrossRef]
- Sharma, M.V.P.; Kumari, D.V.; Subrahmanyam, M. TiO2 supported over SBA-15: An efficient photocatalyst for the pesticide degradation using solar light. Chemosphere 2008, 73, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Agüera, A.; Almansa, E.; Malato, S.; Maldonado, M.I.; Fernández-Alba, A.R. Evaluation of photocatalytic degradation of Imidacloprid in industrial water by GC-MS and LC-MS. Analusis 1998, 26, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Redlich, D.; Shahin, N.; Ekici, P.; Friess, A.; Parlar, H. Kinetical study of the photoinduced degradation of imidacloprid in aquatic media. Clean Soil Air Water 2007, 35, 452–458. [Google Scholar] [CrossRef]
- Kitsiou, V.; Filippidis, N.; Mantzavinos, D.; Poulios, I. Heterogeneous and homogeneous photocatalytic degradation of the insecticide imidacloprid in aqueous solutions. Appl. Catal. B Environ. 2009, 86, 27–35. [Google Scholar] [CrossRef]
- Zang, C.; Zhang, X.; Hu, S.; Chen, F. The role of exposed facets in the Fenton-like reactivity of CeO2 nanocrystal to the Orange II. Appl. Catal. B Environ. 2017, 216, 106–113. [Google Scholar] [CrossRef]
- Yuan, S.; Xu, B.; Zhang, Q.; Liu, S.; Xie, J.; Zhang, M.; Ohno, T. Development of the Visible-Light Response of CeO2-x with a high Ce3+ Content and Its Photocatalytic Properties. ChemCatChem 2018, 10, 1267–1271. [Google Scholar] [CrossRef]
- Xiu, Z.; Xing, Z.; Li, Z.; Wu, X.; Yan, X.; Hu, M.; Cao, Y.; Yang, S.; Zhou, W. Ti3+-TiO2/Ce3+-CeO2 Nanosheet heterojunctions as efficient visible-light driven photocatalysts. Mater. Res. 2018, 100, 191–197. [Google Scholar] [CrossRef]
- Saravanan, R.; Agarwal, S.; Gupta, V.K.; Khan, M.M.; Gracia, F.; Mosquera, E.; Narayanan, V.; Stephen, A. Line defect Ce3+ induced Ag/CeO2/ZnO nanostructure for visible-light photocatalytic activity. J. Photochem. Photobiol. A Chem. 2018, 353, 499–506. [Google Scholar] [CrossRef]
- Černigoj, U.; Štangar, U.L.; Jirkovský, J. Effect of dissolved ozone or ferric ions on photodegradation of thiacloprid in presence of different TiO2 catalysts. J. Hazard. Mater. 2010, 177, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Y.; Lin, T.; He, Y.Y.; Mou, Y.X. Heterogeneous activation of H2O2 by defect-engineered TiO2 − x single crystals for refractory pollutants degradation: A Fenton-like mechanism. J. Hazard. Mater. 2016, 311, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, D.; Shi, W.; Wang, L.; Zhan, J. Yolk-shell structured Fe3O4@void@TiO2 as a photo-Fenton-like catalyst for the extremely efficient elimination of tetracycline. Appl. Catal. B Environ. 2017, 200, 484–492. [Google Scholar] [CrossRef]
- Zhu, X.; Chang, Y.; Chen, Y. Toxicity and bioaccumulation of TiO2 nanoparticle aggregates in Daphnia magna. Chemosphere 2010, 78, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Cantarella, M.; Gorrasi, G.; Di Mauro, A.; Scuderi, M.; Nicotra, G.; Fiorenza, R.; Scirè, S.; Scalisi, M.E.; Brundo, M.V.; Privitera, V.; et al. Mechanical milling: A sustainable route to induce structural transformations in MoS2 for applications in the treatment of contaminated water. Sci. Rep. 2019, 9, 974. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, R.; Bellardita, M.; D’Urso, L.; Compagnini, G.; Palmisano, L.; Scirè, S. Au/TiO2-CeO2 catalysts for photocatalytic water splitting and VOCs oxidation reactions. Catalysts 2016, 6, 121. [Google Scholar] [CrossRef] [Green Version]










| Sample | Ctrl | 10−1 | 10−2 | 10−3 |
|---|---|---|---|---|
| Bare CeO2 | 1.6% (24 h) 6.6% (48 h) | 11.6% (24 h) 28.3% (48 h) | 6.6% (24 h) 23.3% (48 h) | 3.3% (24 h) 21.6% (48 h) |
| Grey CeO2 | 3.3% (24 h) 8.3% (48 h) | 8.3 % (24 h) 23.3% (48 h) | 6.6% (24 h) 18.3% (48 h) | 4.0% (24 h) 15.0% (48 h) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorenza, R.; Balsamo, S.A.; D’Urso, L.; Sciré, S.; Brundo, M.V.; Pecoraro, R.; Scalisi, E.M.; Privitera, V.; Impellizzeri, G. CeO2 for Water Remediation: Comparison of Various Advanced Oxidation Processes. Catalysts 2020, 10, 446. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10040446
Fiorenza R, Balsamo SA, D’Urso L, Sciré S, Brundo MV, Pecoraro R, Scalisi EM, Privitera V, Impellizzeri G. CeO2 for Water Remediation: Comparison of Various Advanced Oxidation Processes. Catalysts. 2020; 10(4):446. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10040446
Chicago/Turabian StyleFiorenza, Roberto, Stefano Andrea Balsamo, Luisa D’Urso, Salvatore Sciré, Maria Violetta Brundo, Roberta Pecoraro, Elena Maria Scalisi, Vittorio Privitera, and Giuliana Impellizzeri. 2020. "CeO2 for Water Remediation: Comparison of Various Advanced Oxidation Processes" Catalysts 10, no. 4: 446. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10040446