A Colorimetric Aptamer Sensor Based on the Enhanced Peroxidase Activity of Functionalized Graphene/Fe3O4-AuNPs for Detection of Lead (II) Ions
Abstract
:1. Introduction
2. Results and Discussion
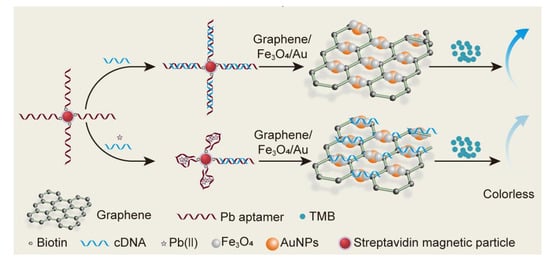
2.1. The Principle of the Colorimetric Assay
2.2. Characterization of Graphene/Fe3O4-AuNP Composites
2.3. Characterization of Amine Magnetic Beads
2.4. Characterization of Streptavidin-Coated Amine Magnetic Beads
2.5. Catalytic Performance Verification of Graphene/Fe3O4−AuNPs
2.6. Optimization of the Concentration of Complementary Strands
2.7. Optimization of the Concentration of Magnetic Beads
2.8. Detection Performances
2.9. Evaluation of Specificity
2.10. Analysis of Pb2+ in Real Samples
3. Materials and Methods
3.1. Chemicals and Apparatus
3.2. Preparation of Graphene/Fe3O4-AuNPs Composites
3.3. Preparation of Amine Magnetic Beads
3.4. Streptavidin Coated Amine Magnetic Beads
3.5. Biotin-Labeled Aptamers Decorated on the Surface of Magnetic Beads
3.6. Evaluation of the Specific Activity
3.7. Detection Procedure of Lead Ion Standards Solution
3.8. Detection of Lead Ions in Real Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gottesfeld, P. The lead battery: A growing global public health challenge. Am. J. Public Health 2017, 107, 1049. [Google Scholar] [CrossRef] [PubMed]
- Zahran, S.; Iverson, T.; McElmurry, S.P.; Weiler, S. The effect of leaded aviation gasoline on blood lead in children. J. Assoc. Environ. Resour. Econ. 2017, 4, 575–610. [Google Scholar] [CrossRef] [Green Version]
- Mariet, A.L.; Sarret, G.; Bégeot, C.; Walter-Simonnet, A.V.; Gimbert, F. Lead highly available in soils centuries after metallurgical activities. J. Environ. Qual. 2017, 46, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Lewis, M. Lead and other heavy metals in soils impacted by exterior legacy paint in residential areas of south west England. Sci. Total Environ. 2018, 619, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Rather, I.A.; Koh, W.Y.; Paek, W.K.; Lim, J. The sources of chemical contaminants in food and their health implications. Front. Pharmacol. 2017, 8, 830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Q.; Narbad, A.; Chen, W. Dietary strategies for the treatment of cadmium and lead toxicity. Nutrients 2015, 7, 552–571. [Google Scholar] [CrossRef] [Green Version]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Carrington, C.D.; Sheehan, D.M.; Bolger, P.M. Hazard assessment of lead. Food Addit. Contam. 1993, 10, 325–335. [Google Scholar] [CrossRef]
- Hauptman, M.; Bruccoleri, R.; Woolf, A.D. An update on childhood lead poisoning. Clin. Pediatr. Emerg. Med. 2017, 18, 181–192. [Google Scholar] [CrossRef]
- Maloney, B.; Bayon, B.L.; Zawia, N.H.; Lahiri, D.K. Latent consequences of early-life lead (Pb) exposure and the future: Addressing the Pb crisis. NeuroToxicology 2018, 68, 126–132. [Google Scholar] [CrossRef]
- Jang, W.H.; Lim, K.M.; Kim, K.; Noh, J.Y.; Kang, S.; Chang, Y.K.; Chung, J.H. Low level of lead can induce phosphatidylserine exposure and erythrophagocytosis: A new mechanism underlying lead-associated anemia. Toxicol. Sci. 2011, 122, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaziri, N.D.; Liang, K.; Ding, Y. Increased nitric oxide inactivation by reactive oxygen species in lead-induced hypertension. Kidney Int. 1999, 56, 1492–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leff, T.; Stemmer, P.; Tyrrell, J.; Jog, R. Diabetes and exposure to environmental lead (Pb). Toxics 2013, 6, 54. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Cui, J.; Chen, Q.; Zhou, N.; Zhou, Z.; Zhang, G.H.; Cao, J. Low-level lead exposure is associated with aberrant sperm quality and reproductive hormone levels in Chinese male individuals: Results from the MARHCS study low-level lead exposure is associated with aberrant sperm quality. Chemosphere 2020, 244, 125402. [Google Scholar] [CrossRef] [PubMed]
- Drinking Water Requirements for States and Public Water. Available online: https://www.epa.gov/dwreginfo/drinking-water-regulations (accessed on 3 May 2020).
- Lead in Food, Foodwares, and Dietary Supplements. Available online: https://www.fda.gov/food/metals-and-your-food/lead-food-foodwares-and-dietary-supplements (accessed on 3 May 2020).
- Ettler, V.; Mihaljevič, M.; Komárek, M. ICP-MS measurements of lead isotopic ratios in soils heavily contaminated by lead smelting: Tracing the sources of pollution. Anal. Bioanal. Chem. 2004, 378, 311–317. [Google Scholar] [CrossRef]
- Candan, N.; Tuzmen, N. Very rapid quantification of malondialdehyde (MDA) in rat brain exposed to lead, aluminium and phenolic antioxidants by high-performance liquid chromatography-fluorescence detection. Neurotoxicology 2008, 29, 708–713. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, T.M.; Peres, J.A.; Felsner, M.L.; Justi, K.C. Direct determination of Pb in raw milk by graphite furnace atomic absorption spectrometry (GF AAS) with electrothermal atomization sampling from slurries. Food Chem. 2017, 229, 721–725. [Google Scholar] [CrossRef]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef]
- Gherase, M.R.; Feng, R.; Fleming, D.E. Optimization of L-shell X-ray fluorescence detection of lead in bone phantoms using synchrotron radiation. X-ray Spectrom. 2017, 46, 537–547. [Google Scholar] [CrossRef]
- Dolati, S.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Recent nucleic acid based biosensors for Pb2+ detection. Sens. Actuators B Chem. 2017, 246, 864–878. [Google Scholar] [CrossRef]
- Boruah, B.S.; Biswas, R. Localized surface plasmon resonance based U-shaped optical fiber probe for the detection of Pb2+ in aqueous medium. Sens. Actuators B Chem. 2018, 276, 89–94. [Google Scholar] [CrossRef]
- Xiong, C.; Liang, W.; Wang, H.; Zheng, Y.; Zhuo, Y.; Chai, Y.; Yuan, R. In situ electro-polymerization of nitrogen doped carbon dots and their application in an electrochemiluminescence biosensor for the detection of intracellular lead ions. Chem. Commun. 2016, 52, 5589–5592. [Google Scholar] [CrossRef] [PubMed]
- Chaiyo, S.; Apiluk, A.; Siangproh, W.; Chailapakul, O. High sensitivity and specificity simultaneous determination of lead, cadmium and copper using μPAD with dual electrochemical and colorimetric detection. Sens. Actuators B Chem. 2016, 233, 540–549. [Google Scholar] [CrossRef]
- Farzin, L.; Shamsipur, M.; Sheibani, S.A. Review: Aptamer-based analytical strategies using the nanomaterials for environmental and human monitoring of toxic heavy metals. Talanta 2017, 174, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Šmuc, T.; Ahn, I.Y.; Ulrich, H. Nucleic acid aptamers as high affinity ligands in biotechnology and biosensorics. J. Pharm. Biomed. Anal. 2013, 81, 210–217. [Google Scholar] [CrossRef]
- Hermann, T.; Patel, D.J. Adaptive recognition by nucleic acid aptamers. Science 2000, 287, 820–825. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; Yang, S. Replacing antibodies with aptamers in lateral flow immunoassay. Biosens. Bioelectron. 2015, 71, 230–242. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, M.; Liu, Y.; Wan, S.; Cui, C.; Zhang, L.; Tan, W. Aptamer/AuNP biosensor for colorimetric profiling of exosomal proteins. Angew. Chem. Int. Ed. 2017, 56, 11916–11920. [Google Scholar] [CrossRef]
- Schoukroun-Barnes, L.R.; Macazo, F.C.; Gutierrez, B.; Lottermoser, J.; Liu, J.; White, R.J. Reagentless, structure-switching, electrochemical aptamer-based sensors. Annu. Rev. Anal. Chem. 2016, 9, 163–181. [Google Scholar] [CrossRef] [Green Version]
- Jepsen, M.D.; Sparvath, S.M.; Nielsen, T.B.; Langvad, A.H.; Grossi, G.; Gothelf, K.V.; Andersen, E.S. Development of a genetically encodable FRET system using fluorescent RNA aptamers. Nat. Commun. 2018, 9, 18. [Google Scholar] [CrossRef]
- Yang, B.; Li, J.; Deng, H.; Zhang, L. Progress of mimetic enzymes and their applications in chemical sensors. Crit. Rev. Anal. Chem. 2016, 46, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Misson, M.; Zhang, H.; Jin, B. Nanobiocatalyst advancements and bioprocessing applications. J. R. Soc. Interface 2015, 12, 20140891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Zhou, Y.; Ren, J.; Qu, X. Carbon nanozymes: Enzymatic properties, catalytic mechanism, and applications. Angew. Chem. Int. Ed. 2018, 57, 9224–9237. [Google Scholar] [CrossRef]
- Jin, S.; Wu, C.; Ye, Z.; Ying, Y. Designed inorganic nanomaterials for intrinsic peroxidase mimics: A review. Sens. Actuators B Chem. 2019, 283, 18–34. [Google Scholar] [CrossRef]
- Duan, D.; Fan, K.; Zhang, D.; Tan, S.; Liang, M.; Liu, Y.; Kobinger, G.P. Nanozyme-strip for rapid local diagnosis of Ebola. Biosens. Bioelectron. 2015, 74, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.Y.; Bu, S.J.; Ju, C.J.; Li, C.T.; Li, Z.Y.; Han, Y.; Wan, J.Y. Hemin-incorporated nanoflowers as enzyme mimics for colorimetric detection of foodborne pathogenic bacteria. Bioorg. Med. Chem. Lett. 2018, 28, 3802–3807. [Google Scholar] [CrossRef]
- Wang, X.; Qin, L.; Zhou, M.; Lou, Z.; Wei, H. Nanozyme sensor arrays for detecting versatile analytes from small molecules to proteins and cells. Anal. Chem. 2018, 90, 11696–11702. [Google Scholar] [CrossRef]
- Nirala, N.R.; Abraham, S.; Kumar, V.; Bansal, A.; Srivastava, A.; Saxena, P.S. Colorimetric detection of cholesterol based on highly efficient peroxidase mimetic activity of graphene quantum dots. Sens. Actuators B Chem. 2015, 218, 42–50. [Google Scholar] [CrossRef]
- Zheng, A.X.; Cong, Z.X.; Wang, J.R.; Li, J.; Yang, H.H.; Chen, G.N. Highly-efficient peroxidase-like catalytic activity of graphene dots for biosensing. Biosens. Bioelectron. 2013, 49, 519–524. [Google Scholar] [CrossRef]
- Garg, B.; Bisht, T.; Ling, Y.C. Graphene-based nanomaterials as efficient peroxidase mimetic catalysts for biosensing applications: An overview. Molecules 2015, 20, 14155–14190. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Zhao, H.; Chen, S.; Yu, H.; Quan, X. Interface engineering catalytic graphene for smart colorimetric biosensing. ACS Nano 2012, 6, 3142–3151. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Zhao, H.; Zang, H.; Ye, F.; Quan, X. Three-dimensional graphene supported bimetallic nanocomposites with DNA regulated-flexibly switchable peroxidase-like activity. ACS Appl. Mater. Interfaces 2016, 8, 9855–9864. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Wang, X.; Zhao, D.; Rex, M.M.; Cho, H.J.; Lee, W.H. A novel nanoporous bismuth electrode sensor for in situ heavy metal detection. Electrochim. Acta 2019, 298, 440–448. [Google Scholar] [CrossRef]
- Satarpai, T.; Shiowatana, J.; Siripinyanond, A. Paper-based analytical device for sampling, on-site preconcentration and detection of ppb lead in water. Talanta 2016, 154, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Yukird, J.; Kongsittikul, P.; Qin, J.; Chailapakul, O.; Rodthongkum, N. ZnO@ graphene nanocomposite modified electrode for sensitive and simultaneous detection of Cd (II) and Pb (II). Synth. Met. 2018, 245, 251–259. [Google Scholar] [CrossRef]
- Li, T.; Dong, S.; Wang, E. A lead (II)-driven DNA molecular device for turn-on fluorescence detection of lead (II) ion with high selectivity and sensitivity. J. Am. Chem. Soc. 2010, 132, 13156–13157. [Google Scholar] [CrossRef]
- Jiang, B.; Duan, D.; Gao, L.; Zhou, M.; Fan, K.; Tang, Y.; Xie, N. Standardized assays for determining the catalytic activity and kinetics of peroxidase-like nanozymes. Nat. Protoc. 2018, 13, 1506–1520. [Google Scholar] [CrossRef]









| Detection Method | LOD (ng/mL) | Linear Range (ng/mL) | Reference |
|---|---|---|---|
| Surface plasmon resonance based fiber probe | 1.2 | 1–20 | [23] |
| Carbon dots based electrochemical sensor | 0.33 | 1–1375 | [24] |
| Microfluidic paper-based sensor | 0.1 | 0.5–70 | [25] |
| Nanoporous bismuth electrode sensor | 1.5 | 5–40 | [45] |
| Paper-based analytical device | 10 | 10–100 | [46] |
| ZnO@graphene modified electrode | 0.8 | 10–200 | [47] |
| Aptamer based colorimetric assay | 0.63 | 1–300 | this work |
| Spiked Samples | Added (ng/mL) | Found (ng/mL) (Graphite Furnace Method) | Found (ng/mL) (This Method) | Spike Recovery (%) |
|---|---|---|---|---|
| 1 | 1 | 1.031 ± 0.024 | 1.06 ± 0.032 | 106.0 |
| 2 | 10 | 10.41 ± 0.33 | 10.58 ± 0.25 | 105.8 |
| 3 | 50 | 48.76 ± 1.01 | 47.63 ± 1.17 | 95.3 |
| 4 | 200 | 208.5 ± 5.57 | 206.6 ± 4.08 | 103.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Z.; Zhou, Y.; Duan, N.; Wang, Z. A Colorimetric Aptamer Sensor Based on the Enhanced Peroxidase Activity of Functionalized Graphene/Fe3O4-AuNPs for Detection of Lead (II) Ions. Catalysts 2020, 10, 600. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10060600
Tao Z, Zhou Y, Duan N, Wang Z. A Colorimetric Aptamer Sensor Based on the Enhanced Peroxidase Activity of Functionalized Graphene/Fe3O4-AuNPs for Detection of Lead (II) Ions. Catalysts. 2020; 10(6):600. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10060600
Chicago/Turabian StyleTao, Zui, You Zhou, Nuo Duan, and Zhouping Wang. 2020. "A Colorimetric Aptamer Sensor Based on the Enhanced Peroxidase Activity of Functionalized Graphene/Fe3O4-AuNPs for Detection of Lead (II) Ions" Catalysts 10, no. 6: 600. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10060600