Enhanced CO2 Methanation Reaction in C1 Chemistry over a Highly Dispersed Nickel Nanocatalyst Prepared Using the One-Step Melt-Infiltration Method
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of the Preparation Conditions on the Ni Dispersion
2.2. Physical and Chemical Properties of Catalysts
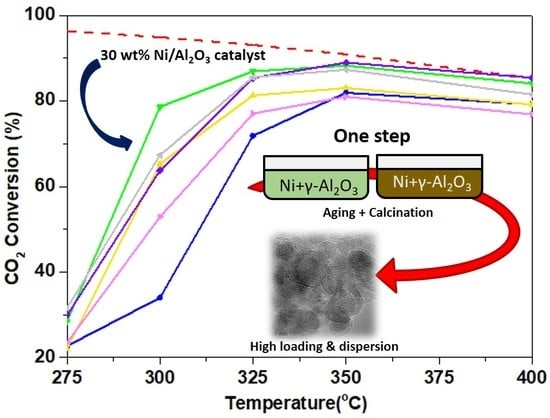
2.3. Catalytic Performance in the CO2 Methanation Reaction
3. Materials and Methods
3.1. Materials
3.2. Preparation of Nickel Catalysts
3.3. Characterization of the Catalysts
3.4. Catalytic Activity Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carrington, G.; Stephenson, J. The politics of energy scenarios: Are International Energy Agency and other conservative projections hampering the renewable energy transition? Energy Res. Soc. Sci. 2018, 46, 103–113. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; Koch, A.M.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef] [Green Version]
- Götz, M.; Koch, A.; Graf, F. State of the art and perspectives of CO2 methanation process concepts for power-to-gas applications, 2014-researchgate.net. In Proceedings of the International Gas Union Research Conference, Copenhagen, Denmark, 17–19 September 2014. Paper Code: TO5-4. [Google Scholar]
- Hashimoto, K.; Yamasaki, M.; Fujimura, K.; Matsui, T.; Izumiya, K.; Komori, M.; El-Moneim, A.; Akiyama, E.; Habazaki, H.; Kumagai, N.; et al. Global CO2 recycling—Novel materials and prospect for prevention of global warming and abundant energy supply. Mater. Sci. Eng. A 1999, 267, 200–206. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—from fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar]
- Swalus, C.; Jacquemin, M.; Poleunis, C.; Bertrand, P.; Ruiz, P. CO2 methanation on Rh/γ-Al2O3 catalyst at low temperature: “In situ” supply of hydrogen by Ni/activated carbon catalyst. Appl. Catal. B Environ. 2012, 125, 41–50. [Google Scholar] [CrossRef]
- Younas, M.; Kong, L.L.; Bashir, M.J.; Nadeem, H.; Shehzad, A.; Sethupathi, S. Recent Advancements, Fundamental Challenges, and Opportunities in Catalytic Methanation of CO2. Energy Fuels 2016, 30, 8815–8831. [Google Scholar] [CrossRef]
- Wang, W.; Gong, J. Methanation of carbon dioxide: An overview. Front. Chem. Sci. Eng. 2011, 5, 2–10. [Google Scholar]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.; Yook, S.; Kim, H. Hydrogen spillover in encapsulated metal catalysts: New opportunities for designing advanced hydroprocessing catalysts. ChemCatChem 2015, 7, 1048–1057. [Google Scholar] [CrossRef]
- Chen, A.; Miyao, T.; Higashiyama, K.; Yamashita, H.; Watanabe, M. High Catalytic Performance of Ruthenium-Doped Mesoporous Nickel–Aluminum Oxides for Selective CO Methanation. Angew. Chem. Int. Ed. 2010, 49, 9895–9898. [Google Scholar] [CrossRef]
- Garbarino, G.; Bellotti, D.; Riani, P.; Magistri, L.; Busca, G. Methanation of carbon dioxide on Ru/Al2O3 and Ni/Al2O3 catalysts at atmospheric pressure: Catalysts activation, behaviour and stability. Int. J. Hydrogen Energy 2015, 40, 9171–9182. [Google Scholar] [CrossRef]
- Karelovic, A.; Ruiz, P. Improving the hydrogenation function of Pd/γ-Al2O3 catalyst by Rh/γ-Al2O3 addition in CO2 methanation at low temperature. ACS Catal. 2013, 3, 2799–2812. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kovarik, L.; Szanyi, J. CO2 reduction on supported Ru/Al2O3 catalysts: Cluster size dependence of product selectivity. ACS Catal. 2013, 3, 2449–2455. [Google Scholar] [CrossRef]
- Park, J.-N.; McFarland, E.W. A highly dispersed Pd–Mg/SiO2 catalyst active for methanation of CO2. J. Catal. 2009, 266, 92–97. [Google Scholar] [CrossRef]
- Sharma, S.; Hu, Z.; Zhang, P.; McFarland, E.W.; Metiu, H. CO2 methanation on Ru-doped ceria. J. Catal. 2011, 278, 297–309. [Google Scholar] [CrossRef]
- Wang, F.; He, S.; Chen, H.; Wang, B.; Zheng, L.; Wei, M.; Evans, D.G.; Duan, X. Active site dependent reaction mechanism over Ru/CeO2 catalyst toward CO2 methanation. J. Am. Chem. Soc. 2016, 138, 6298–6305. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Jalil, A.; Triwahyono, S.; Mukti, R.R.; Taufiq-Yap, Y.; Sazegar, M. Highly active Ni-promoted mesostructured silica nanoparticles for CO2 methanation. Appl. Catal. B Environ. 2014, 147, 359–368. [Google Scholar] [CrossRef]
- Du, G.; Lim, S.; Yang, Y.; Wang, C.; Pfefferle, L.; Haller, G. Methanation of carbon dioxide on Ni-incorporated MCM-41 catalysts: The influence of catalyst pretreatment and study of steady-state reaction. J. Catal. 2007, 249, 370–379. [Google Scholar] [CrossRef]
- Garbarino, G.; Riani, P.; Magistri, L.; Busca, G. A study of the methanation of carbon dioxide on Ni/Al2O3 catalysts at atmospheric pressure. Int. J. Hydrogen Energy 2014, 39, 11557–11565. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; Wang, F.; He, S.; Chen, H.; Zhao, Y.; Wei, M.; Evans, D.G.; Duan, X. Enhanced low-temperature activity of CO2 methanation over highly-dispersed Ni/TiO2 catalyst. Catal. Sci. Technol. 2013, 3, 2627–2633. [Google Scholar] [CrossRef]
- Muroyama, H.; Tsuda, Y.; Asakoshi, T.; Masitah, H.; Okanishi, T.; Matsui, T.; Eguchi, K. Carbon dioxide methanation over Ni catalysts supported on various metal oxides. J. Catal. 2016, 343, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Pan, Q.; Peng, J.; Wang, S.; Wang, S. In situ FTIR spectroscopic study of the CO2 methanation mechanism on Ni/Ce0.5Zr0.5O2. Catal. Sci. Technol. 2014, 4, 502–509. [Google Scholar] [CrossRef]
- Pandey, D.; Deo, G. Effect of support on the catalytic activity of supported Ni–Fe catalysts for the CO2 methanation reaction. J. Ind. Eng. Chem. 2016, 33, 99–107. [Google Scholar] [CrossRef]
- Ren, J.; Qin, X.; Yang, J.-Z.; Qin, Z.-F.; Guo, H.-L.; Lin, J.-Y.; Li, Z. Methanation of carbon dioxide over Ni–M/ZrO2 (M= Fe, Co, Cu) catalysts: Effect of addition of a second metal. Fuel Process. Technol. 2015, 137, 204–211. [Google Scholar] [CrossRef]
- Schild, C.; Wokaun, A.; Baiker, A. On the mechanism of CO and CO2 hydrogenation reactions on zirconia-supported catalysts: A diffuse reflectance FTIR study. J. Mol. Catal. 1990, 63, 243–254. [Google Scholar] [CrossRef]
- Jain, S.K. Density, viscosity, and surface tension of some single molten hydrated salts. J. Chem. Eng. Data 1978, 23, 170–173. [Google Scholar] [CrossRef]
- Eggenhuisen, T.M.; Breejen, J.P.D.; Verdoes, D.; De Jongh, P.E.; De Jong, K.P.D. Fundamentals of melt infiltration for the preparation of supported metal catalysts. The case of Co/SiO2 for Fischer—Tropsch synthesis. J. Am. Chem. Soc. 2010, 132, 18318–18325. [Google Scholar] [CrossRef]
- Ciotonea, C.; Dragoi, B.; Ungureanu, A.; Catrinescu, C.; Petit, S.; Alamdari, H.; Marceau, E.; Dumitriu, E.; Royer, S. Improved dispersion of transition metals in mesoporous materials through a polymer-assisted melt infiltration method. Catal. Sci. Technol. 2017, 7, 5448–5456. [Google Scholar] [CrossRef]
- De Jongh, P.E.; Eggenhuisen, T.M. Melt infiltration: An emerging technique for the preparation of novel functional nanostructured materials. Adv. Mater. 2013, 25, 6672–6690. [Google Scholar] [CrossRef]
- Liu, X.; Khinast, J.G.; Glasser, B.J. Drying of Ni/Alumina catalysts: Control of the metal distribution using surfactants and the melt infiltration method. Ind. Eng. Chem. Res. 2014, 53, 5792–5800. [Google Scholar] [CrossRef]
- Brockner, W.; Ehrhardt, C.; Gjikaj, M. Thermal decomposition of nickel nitrate hexahydrate, Ni(NO3)2·6H2O, in comparison to Co (NO3)2·6H2O and Ca (NO3)2 4H2O. Thermochim. Acta 2007, 456, 64–68. [Google Scholar] [CrossRef]










| Property/Sample | 15Ni/Al2O3 | 20Ni/Al2O3 | 30Ni/Al2O3 | 40Ni/Al2O3 | 50Ni/Al2O3 |
|---|---|---|---|---|---|
| Metal dispersion (%) | 10.3 | 11.44 | 8.7 | 6.7 | 4.2 |
| Metal surface area (m2/gCat) | 10.3 | 15.23 | 17.29 | 17.01 | 13.9 |
| Metal surface area (m2/gNi) | 68.9 | 76.18 | 57.63 | 44.79 | 27.8 |
| Particle diameter (nm) | 9.8 | 8.8 | 11.7 | 15.0 | 24.2 |
| Nickel loading (wt %) a | 14.6 | 21.3 | 30.4 | 38.2 | 46.4 |
| Property/Sample | Fresh-Al2O3 | 15Ni/Al2O3 | 20Ni/Al2O3 | 30Ni/Al2O3 | 40Ni/Al2O3 | 50Ni/Al2O3 |
|---|---|---|---|---|---|---|
| BET surface area (m2/g) | 204.8 | 176.9 | 172.8 | 160.9 | 139.0 | 125.4 |
| Total pore volume (cm3/g) | 0.5 | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 |
| Average pre diameter (nm) | 10.5 | 9.2 | 7.7 | 7.0 | 8.0 | 8.7 |
| Sample | Rate × 10−2 (molCH4 mol Ni−1 s−1) |
|---|---|
| 15Ni/Al2O3 | 9.1 |
| 20Ni/Al2O3 | 12.7 |
| 30Ni/Al2O3 | 8.8 |
| 40Ni/Al2O3 | 4.4 |
| 50Ni/Al2O3 | 3.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, E.H.; Kim, W.; Ko, C.H.; Yoon, W.L. Enhanced CO2 Methanation Reaction in C1 Chemistry over a Highly Dispersed Nickel Nanocatalyst Prepared Using the One-Step Melt-Infiltration Method. Catalysts 2020, 10, 643. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10060643
Cho EH, Kim W, Ko CH, Yoon WL. Enhanced CO2 Methanation Reaction in C1 Chemistry over a Highly Dispersed Nickel Nanocatalyst Prepared Using the One-Step Melt-Infiltration Method. Catalysts. 2020; 10(6):643. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10060643
Chicago/Turabian StyleCho, Eui Hyun, Woohyun Kim, Chang Hyun Ko, and Wang Lai Yoon. 2020. "Enhanced CO2 Methanation Reaction in C1 Chemistry over a Highly Dispersed Nickel Nanocatalyst Prepared Using the One-Step Melt-Infiltration Method" Catalysts 10, no. 6: 643. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10060643