Catalytic Cracking of Heavy Crude Oil over Iron-Based Catalyst Obtained from Galvanic Industry Wastes
Abstract
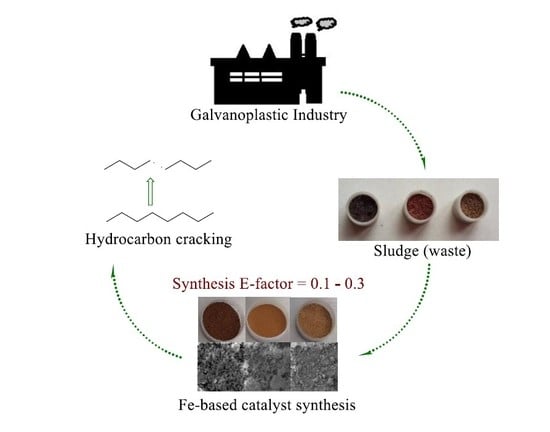
:1. Introduction
2. Results and Discussion
2.1. Sludge and Catalyst Characterization
2.2. Catalyst Reducibility Analysis
2.3. Metallic and Textural Properties of the Catalytic Material
2.4. Catalytic Evaluation of the Synthesized Materials
3. Materials and Methods
3.1. Catalytic Preparation
3.2. Catalyst Characterization
3.3. Catalytic Material Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; Urban Development; World Bank: Washington, DC, USA, 2018. [Google Scholar]
- Mymrin, V.; Borgo, S.C.; Alekseev, K.; Avanci, M.A.; Rolim, P.H.B.; Argenda, M.A.; Klitzke, W.; Gonçalves, A.J.; Catai, R.E. Galvanic Cr-Zn and spent foundry sand waste application as valuable components of sustainable ceramics to prevent environment pollution. Int. J. Adv. Manuf. Technol. 2020, 107, 1239–1250. [Google Scholar] [CrossRef]
- Pérez-Villarejo, L.; Martínez-Martínez, S.; Carrasco-Hurtado, B.; Eliche-Quesada, D.; Ureña-Nieto, C.; Sánchez-Soto, P.J. Valorization and inertization of galvanic sludge waste in clay bricks. Appl. Clay Sci. 2015, 105–106, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Chen, L.; Chen, D.; Zhou, S. Characteristics and aluminum reuse of textile sludge incineration residues after acidification. J. Environ. Sci. 2011, 23, 1999–2004. [Google Scholar] [CrossRef]
- Sohaimi, K.S.A.; Ngadi, N.; Mat, H.; Inuwa, I.M.; Wong, S. Synthesis, characterization and application of textile sludge biochars for oil removal. J. Environ. Chem. Eng. 2017, 5, 1415–1422. [Google Scholar] [CrossRef]
- Cagno, E.; Trucco, P. Cleaner technology transfer in the Italian galvanic industry: Economic and know-how issues. J. Clean. Prod. 2008, 16, S32–S36. [Google Scholar] [CrossRef]
- Stepanov, S.; Morozov, N.; Morozova, N.; Ayupov, D.; Makarov, D.; Baishev, D. Efficiency of Use of Galvanic Sludge in Cement Systems. Procedia Eng. 2016, 165, 1112–1117. [Google Scholar] [CrossRef]
- Svoboda, K.; Baxter, D.; Martinec, J. Nitrous oxide emissions from waste incineration. Chem. Pap. 2006, 60, 78–90. [Google Scholar] [CrossRef]
- Werle, S. A reburning process using sewage sludge-derived syngas. Chem. Pap. 2012, 66, 99–107. [Google Scholar] [CrossRef]
- Rossini, G.; Bernardes, A.M. Galvanic sludge metals recovery by pyrometallurgical and hydrometallurgical treatment. J. Hazard. Mater. 2006, 131, 210–216. [Google Scholar] [CrossRef]
- Cichowicz, R.; Stelęgowski, A. Effect of thermal sludge processing on selected components of air quality in the vicinity of a wastewater treatment plant. Chem. Pap. 2019, 73, 843–849. [Google Scholar] [CrossRef]
- Castañeda Bocanegra, J.J.; Espejo Mora, E.; Cubillos González, G.I. Encapsulation in ceramic material of the metals Cr, Ni, and Cu contained in galvanic sludge via the solidification/stabilization method. J. Environ. Chem. Eng. 2017, 5, 3834–3843. [Google Scholar] [CrossRef]
- Felisberto, R.; Santos, M.C.; Arcaro, S.; Basegio, T.M.; Bergmann, C.P. Assessment of environmental compatibility of glass–ceramic materials obtained from galvanic sludge and soda–lime glass residue. Process Saf. Environ. Prot. 2018, 120, 72–78. [Google Scholar] [CrossRef]
- Luz, C.A.; Rocha, J.C.; Cheriaf, M.; Pera, J. Valorization of galvanic sludge in sulfoaluminate cement. Constr. Build. Mater. 2009, 23, 595–601. [Google Scholar] [CrossRef]
- Bednarik, V.; Vondruska, M.; Koutny, M. Stabilization/solidification of galvanic sludges by asphalt emulsions. J. Hazard. Mater. 2005, 122, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Yac’cob, N.A.N.; Ngadi, N.; Hassan, O.; Inuwa, I.M. From pollutant to solution of wastewater pollution: Synthesis of activated carbon from textile sludge for dye adsorption. Chin. J. Chem. Eng. 2018, 26, 870–878. [Google Scholar] [CrossRef]
- Amaral, F.A.D.; dos Santos, V.S.; Bernardes, A.M. Metals recovery from galvanic sludge by sulfate roasting and thiosulfate leaching. Miner. Eng. 2014, 60, 1–7. [Google Scholar] [CrossRef]
- Huyen, P.T.; Dang, T.D.; Tung, M.T.; Huyen, N.T.T.; Green, T.A.; Roy, S. Electrochemical copper recovery from galvanic sludge. Hydrometallurgy 2016, 164, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Jandová, J.; Štefanová, T.; Niemczyková, R. Recovery of Cu-concentrates from waste galvanic copper sludges. Hydrometallurgy 2000, 57, 77–84. [Google Scholar] [CrossRef]
- Silva, J.E.; Paiva, A.P.; Soares, D.; Labrincha, A.; Castro, F. Solvent extraction applied to the recovery of heavy metals from galvanic sludge. J. Hazard. Mater. 2005, 120, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Klose, F.; Scholz, P.; Kreisel, G.; Ondruschka, B.; Kneise, R.; Knopf, U. Catalysts from waste materials. Appl. Catal. B Environ. 2000, 28, 209–221. [Google Scholar] [CrossRef]
- Sushil, S.; Scholz, P.; Pollok, K.; Ondruschka, B.; Batra, V.S. Application of industrial waste based catalysts for total oxidation of propane. Chem. Eng. J. 2011, 166, 568–578. [Google Scholar] [CrossRef]
- Lou, J.-C.; Chang, C.-K. Catalytic Oxidation of CO Over a Catalyst Produced in the Ferrite Process. Environ. Eng. Sci. 2006, 23, 1024–1032. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, J.; Xu, Y.; Su, H.; Li, X.; Zhou, J.Z.; Qian, G.; Li, L.; Xu, Z.P. Efficient Selective Catalytic Reduction of NO by Novel Carbon-doped Metal Catalysts Made from Electroplating Sludge. Environ. Sci. Technol. 2014, 48, 11497–11503. [Google Scholar] [CrossRef] [PubMed]
- Montero, C.; Castañeda, K.M.; Suntasig, Y.M.O.; Oña, D.R.F.; De La Rosa, A. Catalyst Based on Sludge Derived from Wastewater Treatment of Textile Industry. Chem. Eng. Trans. 2018, 70, 931–936. [Google Scholar] [CrossRef]
- Lin, B.; Huang, Q.; Yang, Y.; Chi, Y. Preparation of Fe-char catalyst from tank cleaning oily sludge for the catalytic cracking of oily sludge. J. Anal. Appl. Pyrolysis 2019, 139, 308–318. [Google Scholar] [CrossRef]
- Lee, M.S.; Park, K.Y.; Park, H.K.; Kang, T.W.; Jang, H.D.; Han, S.S.; Jeon, J.-K. Prospective application of carbon-silica derived from SiC-Si sludge as a support for Fe catalysts. Korean J. Chem. Eng. 2017, 34, 100–104. [Google Scholar] [CrossRef]
- Nam, S.-B.; Park, Y.-S.; Yun, Y.-S.; Gu, J.-H.; Sung, H.-J.; Horio, M. Catalytic application of metallic iron from the dyeing sludge ash for benzene steam reforming reaction in tar emitted from biomass gasification. Korean J. Chem. Eng. 2016, 33, 465–472. [Google Scholar] [CrossRef]
- Zhu, F.; Jiang, H.; Zhang, Z.; Zhao, L.; Wang, J.; Hu, J.; Zhang, H. Research on Drying Effect of Different Additives on Sewage Sludge. Procedia Environ. Sci. 2012, 16, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Ronda, A.; Gómez-Barea, A.; Haro, P.; de Almeida, V.F.; Salinero, J. Elements partitioning during thermal conversion of sewage sludge. Fuel Process. Technol. 2019, 186, 156–166. [Google Scholar] [CrossRef]
- Guangyin, Z.; Youcai, Z. Chapter Three—Sewage Sludge Solidification/Stabilization and Drying/Incineration Process. In Pollution Control and Resource Recovery for Sewage Sludge; Guangyin, Z., Youcai, Z., Eds.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 101–160. ISBN 978-0-12-811639-5. [Google Scholar]
- Kuzin, E.N.; Chernyshev, P.I.; Vizen, N.S.; Krutchinina, N.E. The Purification of the Galvanic Industry Wastewater of Chromium(VI) Compounds Using Titanium(III) Chloride. Russ. J. Gen. Chem. 2018, 88, 2954–2957. [Google Scholar] [CrossRef]
- Kliopova, I.; Staniškis, J. Optimization of Galvanic Wastewater Treatment Processes. In Modern Tools and Methods of Water Treatment for Improving Living Standards; Omelchenko, A., Pivovarov, A.A., Swindall, W.J., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 197–208. [Google Scholar]
- Torres-Luna, J.A.; Carriazo, J.G.; Sanabria-González, N.R. Calcination Temperature Effect on structural and textural properties of Fe(iii)-TiO2. Rev. Fac. Cienc. Básicas 2014, 10, 186–195. [Google Scholar] [CrossRef]
- Sheldon, R.A. Fundamentals of green chemistry: Efficiency in reaction design. Chem. Soc. Rev. 2012, 41, 1437–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dicks, A.P.; Hent, A. The E Factor and Process Mass Intensity. In Green Chemistry Metrics: A Guide to Determining and Evaluating Process Greenness; SpringerBriefs in Molecular Science; Dicks, A.P., Hent, A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 45–67. ISBN 978-3-319-10500-0. [Google Scholar]
- Tieves, F.; Tonin, F.; Fernández-Fueyo, E.; Robbins, J.M.; Bommarius, B.; Bommarius, A.S.; Alcalde, M.; Hollmann, F. Energising the E-factor: The E+-factor. Tetrahedron 2019, 75, 1311–1314. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Agarwal, K.; Singh, A.K.; Polke, B.G.; Raha, K.C. Characterization of γ- and α-Fe2O3 nano powders synthesized by emulsion precipitation-calcination route and rheological behaviour of α-Fe2O3. Int. J. Eng. Sci. Technol. 2010, 2. [Google Scholar] [CrossRef] [Green Version]
- Ortego, J.D.; Barroeta, Y.; Cartledge, F.K.; Akhter, H. Leaching effects on silicate polymerization. An FTIR and silicon-29 NMR study of lead and zinc in portland cement. Environ. Sci. Technol. 1991, 25, 1171–1174. [Google Scholar] [CrossRef]
- Paterson, E. The Iron Oxides. Structure, Properties, Reactions, Occurrences and Uses. Clay Min. 2006, 34, 209–210. [Google Scholar] [CrossRef]
- Iqbal, A.; Jacob, J.; Mahmood, A.; Mehboob, K.; Mahmood, K.; Ali, A.; Bukhari, T.H.; Adrees, M.; Ibrahim, M.; Ahmad, M. Synthesis and characterization of Zn–Mn–Fe nano oxide composites for the degradation of reactive yellow 15 dye. Phys. B Condens. Matter 2020, 588, 412210. [Google Scholar] [CrossRef]
- Fakeeha, A.; Khan, W.; Ibrahim, A.; Al-Otaibi, R.; Alfatesh, A.; Soliman, M.; Abasaeed, A. Alumina supported iron catalyst for hydrogen production: Calcination study. Int. J. Adv. Chem. Eng. Biol. Sci. 2015, 2, 139–141. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Li, J.; Hu, Y.; Wang, W.; Yuan, D.; Wang, Y.; Yang, T.; Li, L.; Sun, H.; et al. Promotion of the Inactive Iron Sulfide to an Efficient Hydrodesulfurization Catalyst. ACS Catal. 2017, 7, 4805–4816. [Google Scholar] [CrossRef]
- Liang, K.; Zhang, C.; Xiang, H.; Yang, Y.; Li, Y. Effects of modified SiO2 on H2 and CO adsorption and hydrogenation of iron-based catalysts. J. Fuel Chem. Technol. 2019, 47, 769–779. [Google Scholar] [CrossRef]
- Song, H.; Cui, H.; Li, F. The effect of Zn–Fe modified S2O8 2−/ZrO2–Al2O3 catalyst for n-pentane hydroisomerization. Res. Chem. Intermed. 2016, 42, 3029–3038. [Google Scholar] [CrossRef]
- National Institute for Materials Science NIMS Materials Database (MatNavi). Available online: https://mits.nims.go.jp/index_en.html (accessed on 12 May 2020).
- Glavee, G.N.; Klabunde, K.J.; Sorensen, C.M.; Hadjipanayis, G.C. Chemistry of Borohydride Reduction of Iron(II) and Iron(III) Ions in Aqueous and Nonaqueous Media. Formation of Nanoscale Fe, FeB, and Fe2B Powders. Inorg. Chem. 1995, 34, 28–35. [Google Scholar] [CrossRef]
- Legodi, M.A.; de Waal, D. The preparation of magnetite, goethite, hematite and maghemite of pigment quality from mill scale iron waste. Dyes Pigments 2007, 74, 161–168. [Google Scholar] [CrossRef]
- Picasso, G.; Sun Kou, R.; Gómez, G.; Hermoza, E.; López, A.; Pina, M.P.; Herguido, J. Nanosized catalyst based on Fe Oxide for combustion of n-hexane. Rev. Soc. Quím. Perú 2009, 75, 163–176. [Google Scholar]
- Caballero, D.; Mass, J.; Landinez, D. Optical and Structural Characterization of Zn2 TiO4 capped with Mg. Tumbaga 2011, 6, 165–172. [Google Scholar]
- Kumar, H.; Rani, R. Structural and Optical Characterization of ZnO Nanoparticles Synthesized by Microemulsion Route. Int. Lett. Chem. Phys. Astron. 2013, 14, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Dou, J.; Li, X.; Tahmasebi, A.; Xu, J.; Yu, J. Desulfurization of coke oven gas using char-supported Fe-Zn-Mo catalysts: Mechanisms and thermodynamics. Korean J. Chem. Eng. 2015, 32, 2227–2235. [Google Scholar] [CrossRef]
- Yan, Z.; Kang, Y.; Li, D.; Liu, Y.C. Catalytic oxidation of sulfur dioxide over α-Fe2O3/SiO2 catalyst promoted with Co and Ce oxides. Korean J. Chem. Eng. 2020, 37, 623–632. [Google Scholar] [CrossRef]
- Yin, X.; Yue, M.; Lu, Q.; Liu, M.; Wang, F.; Qiu, Y.; Liu, W.; Zuo, T.; Zha, S.; Li, X.; et al. An Efficient Process for Recycling Nd–Fe–B Sludge as High-Performance Sintered Magnets. Engineering 2020, 6, 165–172. [Google Scholar] [CrossRef]
- Kök, M.V.; Varfolomeev, M.A.; Nurgaliev, D.K. Crude oil characterization using TGA-DTA, TGA-FTIR and TGA-MS techniques. J. Pet. Sci. Eng. 2017, 154, 537–542. [Google Scholar] [CrossRef]
- Simo, S.M.; Naman, S.A.; Ahmed, K.R.; Faritovich, A.A. Evaluation of Two Kurdistan-Iraq Crude Oil (T-21A, PF2) by Derivatographic Method. Int. Res. J. Pure Appl. Chem. 2020, 38–46. [Google Scholar] [CrossRef]
- Park, Y.C.; Paek, J.-Y.; Bae, D.-H.; Shun, D. Study of pyrolysis kinetics of Alberta oil sand by thermogravimetric analysis. Korean J. Chem. Eng. 2009, 26, 1608–1612. [Google Scholar] [CrossRef]
- Liu, S.; Ren, J.; Zhu, S.; Zhang, H.; Lv, E.; Xu, J.; Li, Y.-W. Synthesis and characterization of the Fe-substituted ZSM-22 zeolite catalyst with high n-dodecane isomerization performance. J. Catal. 2015, 330, 485–496. [Google Scholar] [CrossRef]






| Sample | T, °C | % N | % C | % H | % S | Surface Area, m2·g−1 | Pore Volume, cm3·g−1 | E-Factor |
|---|---|---|---|---|---|---|---|---|
| L1 | 120 | 0.10 | 2.36 | 2.05 | 0.94 | nm | nm | nm |
| C1 | 400 | 0.02 | 0.65 | 0.35 | 0.39 | 96.15 | 0.12 | 0.1 |
| 500 | 0.05 | 0.44 | 0.19 | 0.38 | 71.37 | 0.10 | ||
| 700 | 0.03 | 0.16 | 0.08 | 0.35 | 14.98 | 0.02 | ||
| L2 | 120 | 0.15 | 6.48 | 0.52 | 0.20 | nm | nm | nm |
| C2 | 400 | 0.02 | 5.48 | 0.18 | 0.22 | 73.46 | 0.10 | 0.1 |
| 500 | 0.03 | 5.48 | 0.14 | 0.23 | 63.36 | 0.09 | ||
| 700 | 0.01 | 4.77 | 0.08 | 0.20 | 27.56 | 0.03 | ||
| L3 | 100 | 1.11 | 5.86 | 1.35 | 2.94 | nm | nm | nm |
| C3 | 400 | 0.04 | 1.74 | 0.08 | 3.71 | 29.72 | 0.03 | 0.3 |
| 500 | 0.03 | 0.40 | 0.08 | 4.23 | 17.68 | 0.02 | ||
| 700 | 0.02 | 0.20 | 0.06 | 4.42 | 4.53 | 0.01 |
| Temperature of Reaction, °C | Sample | Weight Loss,% | Kinetic Expression | R2 | Rate of Mass Loss (avg.), mg·min−1 |
|---|---|---|---|---|---|
| 400 | Crude oil without catalyst | 54.30 | 0.90 | 0.57 | |
| Crude oil + C1 | 59.28 | 0.92 | 0.61 | ||
| Crude oil + commercial cat. | 56.00 | 0.95 | 0.65 | ||
| 450 | Crude oil without catalyst | 73.20 | 0.91 | 0.64 | |
| Crude oil + C2 | 84.50 | 0.90 | 0.71 | ||
| Crude oil + C3 | 82.10 | 0.92 | 0.69 | ||
| Crude oil + commercial cat. | 77.95 | 0.94 | 0.81 |
| Company | Sludge | Catalytic Material |
|---|---|---|
| Company 1 | L1 | C1 |
| Company 2 | L2 | C2 |
| Company 3 | L3 | C3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villamarin-Barriga, E.; Canacuán, J.; Londoño-Larrea, P.; Solís, H.; De La Rosa, A.; Saldarriaga, J.F.; Montero, C. Catalytic Cracking of Heavy Crude Oil over Iron-Based Catalyst Obtained from Galvanic Industry Wastes. Catalysts 2020, 10, 736. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10070736
Villamarin-Barriga E, Canacuán J, Londoño-Larrea P, Solís H, De La Rosa A, Saldarriaga JF, Montero C. Catalytic Cracking of Heavy Crude Oil over Iron-Based Catalyst Obtained from Galvanic Industry Wastes. Catalysts. 2020; 10(7):736. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10070736
Chicago/Turabian StyleVillamarin-Barriga, Estefanía, Jéssica Canacuán, Pablo Londoño-Larrea, Hugo Solís, Andrés De La Rosa, Juan F. Saldarriaga, and Carolina Montero. 2020. "Catalytic Cracking of Heavy Crude Oil over Iron-Based Catalyst Obtained from Galvanic Industry Wastes" Catalysts 10, no. 7: 736. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10070736