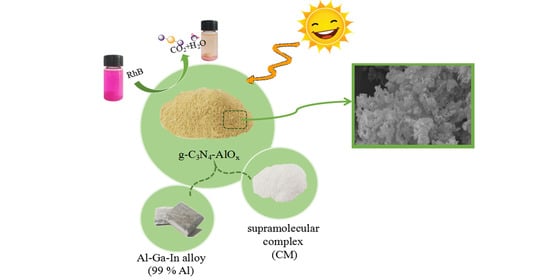
The Preparation of Amorphous Aluminum Oxide Modified g-C3N4 to Improve Photocatalytic Performance in Contaminant Degradation Applications
Abstract
:1. Introduction
2. Result and Discussion
2.1. Characterization of As-Prepared Samples
2.2. Photocatalytic Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of Photocatalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dai, L.; Chang, D.W.; Baek, J.B.; Lu, W. Carbon nanomaterials for advanced energy conversion and storage. Small 2012, 8, 1130–1166. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Qu, F.; Tian, L.; Yang, X.; Zou, Z.; Lin, Z. Self-assembled g-C3N4 nanoarchitectures with boosted photocatalytic solar-to-hydrogen efficiency. Appl. Surf. Sci. 2019, 487, 59–67. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, B.; Wang, H.; Yang, Y.; Zhu, H.; Yu, W.; Basset, J.M. Surface modification of g-C3N4 by hydrazine: Simple way for noble-metal free hydrogen evolution catalysts. Chem. Eng. J. 2016, 286, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Li, F.T.; Xue, Y.B.; Li, B.; Hao, Y.J.; Wang, X.J.; Liu, R.H.; Zhao, J. Precipitation Synthesis of Mesoporous Photoactive Al2O3 for Constructing g-C3N4-Based Heterojunctions with Enhanced Photocatalytic Activity. Ind. Eng. Chem. Res. 2014, 53, 19540–19549. [Google Scholar] [CrossRef]
- Li, F.T.; Zhao, Y.; Wang, Q.; Wang, X.J.; Hao, Y.J.; Liu, R.H.; Zhao, D. Enhanced visible-light photocatalytic activity of active Al2O3/g-C3N4 heterojunctions synthesized via surface hydroxyl modification. J. Hazard. Mater. 2015, 283, 371–381. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, T.; Li, B.; Jiang, S.; Zhang, X.; Hai, L.; Chen, X.; Wu, W. An ingenious strategy of preparing TiO2/g-C3N4 heterojunction photocatalyst: In situ growth of TiO2 nanocrystals on g-C3N4 nanosheets via impregnation-calcination method. Appl. Surf. Sci. 2018, 433, 963–974. [Google Scholar] [CrossRef]
- Chang, F.; Zhang, J.; Xie, Y.; Chen, J.; Li, C.; Wang, J.; Luo, J.; Deng, B.; Hu, X. Fabrication, characterization, and photocatalytic performance of exfoliated g-C3N4–TiO2 hybrids. Appl. Surf. Sci. 2014, 311, 574–581. [Google Scholar] [CrossRef]
- Kamegawa, T.; Matsuura, S.; Seto, H.; Yamashita, H. A visible-light-harvesting assembly with a sulfocalixarene linker between dyes and a Pt-TiO2 photocatalyst. Angew. Chem. Int. Ed. Engl. 2013, 52, 916–919. [Google Scholar] [CrossRef]
- Yang, J.H.; Wang, D.G.; Han, H.X.; Li, C. Roles of Cocatalysts in Photocatalysis and Photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef]
- Cao, S.; Yu, J. g-C3N4-Based Photocatalysts for Hydrogen Generation. J. Phys. Chem. Lett. 2014, 5, 2101–2107. [Google Scholar] [CrossRef]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric Graphitic Carbon Nitride for Heterogeneous Photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Butchosa, C.; Guiglion, P.; Zwijnenburg, M.A. Carbon Nitride Photocatalysts for Water Splitting: A Computational Perspective. J. Phys. Chem. C 2014, 118, 24833–24842. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Z.; Bai, H.; Sun, D.D. Concurrent filtration and solar photocatalytic disinfection/degradation using high-performance Ag/TiO2 nanofiber membrane. Water Res. 2012, 46, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hu, M.; Mi, B. Membrane surface modification with TiO2–graphene oxide for enhanced photocatalytic performance. J. Membr. Sci. 2014, 455, 349–356. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, Z.; Xiong, T.; Ni, Z.; Zhang, W.; Sun, Y.; Ho, W.K. In situ construction of g-C3N4/g-C3N4 metal-free heterojunction for enhanced visible-light photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 11392–11401. [Google Scholar] [CrossRef]
- Sun, Y.F.; Cheng, H.; Gao, S.; Sun, Z.H.; Liu, Q.H.; Liu, Q.; Lei, F.C.; Yao, T.; He, J.F.; Wei, S.Q.; et al. Freestanding Tin Disulfide Single-Layers Realizing Efficient Visible-Light Water Splitting. Angew. Chem. Int. Ed. 2012, 51, 8727–8731. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Lei, Y.; Wu, N.; Gou, Y.; Han, C.; Xie, S.; Fang, D. Mesoporous silicon carbide nanofibers with in situ embedded carbon for co-catalyst free photocatalytic hydrogen production. Nano Res. 2016, 9, 886–898. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Y.; Li, B.; Li, A.; Yang, P.; Yang, L.; Wang, G.; Chen, J.; Wang, R. In-situ hydrothermal synthesized γ-Al2O3/O-g-C3N4 heterojunctions with enhanced visible-light photocatalytic activity in water splitting for hydrogen. J. Energy Chem. 2016, 25, 594–600. [Google Scholar] [CrossRef]
- Lai, S.H.; Chen, Y.B.; Li, N.; Su, H.; Guo, S.H. Novel g-C3N4 wrapped γ-Al2O3 microspheres heterojunction for efficient photocatalytic application under visible light irradiation. J. Mater. Sci. Mater. Electron. 2017, 29, 4509–4516. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Sagara, N.; Kamimura, S.; Tsubota, T.; Ohno, T. Photoelectrochemical CO2 reduction by a p-type boron-doped g-C3N4 electrode under visible light. Appl. Catal. B Environ. 2016, 192, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Schultz, D.M.; Yoon, T.P. Solar synthesis: Prospects in visible light photocatalysis. Science 2014, 343, 1239176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Su, F.; Mathew, S.C.; Lipner, G.; Fu, X.; Antonietti, M.; Blechert, S.; Wang, X. mpg-C3N4-Catalyzed Selective Oxidation of Alcohols Using O2 and Visible Light. J. Am. Chem. Soc. 2010, 132, 16299–16301. [Google Scholar] [CrossRef]
- Ye, C.; Li, J.X.; Li, Z.J.; Li, X.B.; Fan, X.B.; Zhang, L.P.; Chen, B.; Tung, C.-H.; Wu, L.Z. Enhanced Driving Force and Charge Separation Efficiency of Protonated g-C3N4 for Photocatalytic O2 Evolution. ACS Catal. 2015, 5, 6973–6979. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.; Maeda, K.; Domen, K.; Liu, P.; Antonietti, M.; Fu, X.; Wang, X. Sulfur-mediated synthesis of carbon nitride: Band-gap engineering and improved functions for photocatalysis. Energy Environ. Sci. 2011, 4, 675–678. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, X. A Review on Quantum Dots Modified g-C3N4-Based Photocatalysts with Improved Photocatalytic Activity. Catalysts 2020, 10, 142. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, G.; Chen, S.; Fan, X.; Quan, X.; Yu, H. Integration of membrane filtration and photoelectrocatalysis on g-C3N4/CNTs/Al2O3 membrane with visible-light response for enhanced water treatment. J. Membr. Sci. 2017, 541, 153–161. [Google Scholar] [CrossRef]
- Zhang, X.S.; Hu, J.Y.; Jiang, H. Facile modification of a graphitic carbon nitride catalyst to improve its photoreactivity under visible light irradiation. Chem. Eng. J. 2014, 256, 230–237. [Google Scholar] [CrossRef]
- Zhang, G.; Lan, Z.A.; Lin, L.; Lin, S.; Wang, X. Overall water splitting by Pt/g-C3N4 photocatalysts without using sacrificial agents. Chem. Sci. 2016, 7, 3062–3066. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, N.; Zhao, F.; Liu, T.; Wang, Y. Facile Fabrication of a Novel Au/Phosphorus-Doped g-C3N4 Photocatalyst with Excellent Visible Light Photocatalytic Activity. Catalysts 2020, 10, 701. [Google Scholar] [CrossRef]
- Min, S.; Lu, G. Enhanced Electron Transfer from the Excited Eosin Y to mpg-C3N4 for Highly Efficient Hydrogen Evolution under 550 nm Irradiation. J. Phys. Chem. C 2012, 116, 19644–19652. [Google Scholar] [CrossRef]
- Goettmann, F.; Fischer, A.; Antonietti, M.; Thomas, A. Chemical synthesis of mesoporous carbon nitrides using hard templates and their use as a metal-free catalyst for Friedel-Crafts reaction of benzene. Angew. Chem. Int. Ed. Engl. 2006, 45, 4467–4471. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Gong, Y.; Zhang, J.; Zhan, L.; Ma, L.; Fang, Z.; Vajtai, R.; Wang, X.; Ajayan, P.M. Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light. Adv. Mater. 2013, 25, 2452–2456. [Google Scholar] [CrossRef]
- Dong, G.; Zhao, K.; Zhang, L. Carbon self-doping induced high electronic conductivity and photoreactivity of g-C3N4. Chem. Commun. 2012, 48, 6178–6180. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation of rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation. Langmuir 2010, 26, 3894–3901. [Google Scholar] [CrossRef]
- Ji, C.; Yin, S.N.; Sun, S.; Yang, S. An in situ mediator-free route to fabricate Cu2O/g-C3N4 type-II heterojunctions for enhanced visible-light photocatalytic H2 generation. Appl. Surf. Sci. 2018, 434, 1224–1231. [Google Scholar] [CrossRef]
- Wang, H.; Bai, J.; Dai, M.; Liu, K.; Liu, Y.; Zhou, L.; Liu, F.; Liu, F.; Gao, Y.; Yan, X.; et al. Visible light activated excellent NO2 sensing based on 2D/2D ZnO/g-C3N4 heterojunction composites. Sens. Actuators B Chem. 2020, 304, 127287. [Google Scholar] [CrossRef]
- Chen, X.; Hu, S.Z.; Li, P.; Li, W.; Ma, H.F.; Lu, G. Photocatalytic Production of Hydrogen Peroxide Using g-C3N4 Coated MgO-Al2O3-Fe2O3 Heterojunction Catalysts Prepared by a Novel Molten Salt-Assisted Microwave Process. Acta Phys.-Chim. Sin. 2017, 33, 2532–2541. [Google Scholar]
- Song, J.; Wang, X.; Ma, J.; Wang, X.; Wang, J.; Zhao, J. Visible-light-driven in situ inactivation of Microcystis aeruginosa with the use of floating g-C3N4 heterojunction photocatalyst: Performance, mechanisms and implications. Appl. Catal. B Environ. 2018, 226, 83–92. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Wang, P.; Hou, J.; Wang, C.; Ao, Y. Robust photocatalytic hydrogen evolution over amorphous ruthenium phosphide quantum dots modified g-C3N4 nanosheet. Appl. Catal. B Environ. 2018, 239, 578–585. [Google Scholar] [CrossRef]
- Lan, Y.; Li, Z.; Xie, W.; Li, D.; Yan, G.; Guo, S.; Pan, C.; Wu, J. In situ fabrication of I-doped Bi2O2CO3/g-C3N4 heterojunctions for enhanced photodegradation activity under visible light. J. Hazard. Mater. 2020, 385, 121622. [Google Scholar] [CrossRef] [PubMed]
- Tallapally, V.; Damma, D.; Darmakkolla, S.R. Facile synthesis of size-tunable tin arsenide nanocrystals. Chem. Commun. 2019, 55, 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Holger, B.; Elena, V.S.; Aymeric, R.; Ivo, M.; Andreas, K.; Gerhard, G.; Horst, W. Determination of Nanocrystal Sizes: A Comparison of TEM, SAXS, and XRD Studies of Highly Monodisperse CoPt3 Particles. Langmuir 2005, 21, 1931–1936. [Google Scholar]







| Element | Weight% | Atomic% |
|---|---|---|
| C | 39.61 | 44.19 |
| N | 51.52 | 49.30 |
| Al | 2.68 | 1.33 |
| Sample | Fitted Equation | k (min−1) | Correlation Coefficient R |
|---|---|---|---|
| no catalyst | ln(C0/Ct) = 1.96 × 10−4t + 3.37 × 10−4 | 1.96 × 10−4 | 9.89 × 10−1 |
| g-C3N4 | ln(C0/Ct) = 1.42 × 10−2t − 5.39 × 10−2 | 1.42 × 10−2 | 9.94 × 10−1 |
| g-C3N4-AlOx | ln(C0/Ct) = 2.92 × 10−2t − 1.10 × 10−1 | 2.92 × 10−2 | 9.94 × 10−1 |
| g-C3N4-AlOx-adsorption | ln(C0/Ct) = 1.01 × 10−3t + 3.52 × 10−1 | 1.01 × 10−3 | 9.97 × 10−1 |
| amorphous aluminum oxide | ln(C0/Ct) = 4.89 × 10−4t + 3.34 × 10−2 | 4.89 × 10−4 | 9.92 × 10−1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Wang, C.; Fu, W.; An, Q.; Wei, C.; Li, L. The Preparation of Amorphous Aluminum Oxide Modified g-C3N4 to Improve Photocatalytic Performance in Contaminant Degradation Applications. Catalysts 2020, 10, 1036. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091036
Zheng Y, Wang C, Fu W, An Q, Wei C, Li L. The Preparation of Amorphous Aluminum Oxide Modified g-C3N4 to Improve Photocatalytic Performance in Contaminant Degradation Applications. Catalysts. 2020; 10(9):1036. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091036
Chicago/Turabian StyleZheng, Yining, Congcong Wang, Wenjing Fu, Qi An, Cundi Wei, and Lina Li. 2020. "The Preparation of Amorphous Aluminum Oxide Modified g-C3N4 to Improve Photocatalytic Performance in Contaminant Degradation Applications" Catalysts 10, no. 9: 1036. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091036