Application of Activated Carbon to Obtain Biodiesel from Vegetable Oils
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Activated Carbon
2.2. Effect of the Production Process Parameters on the Yield of Biodiesel
2.3. The Effect of Various Sources of TAGs on Biodiesel Properties
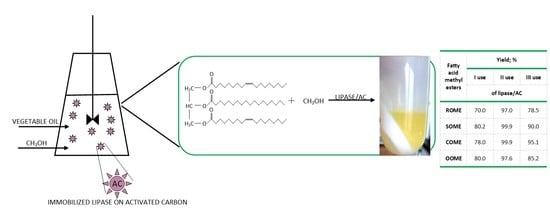
2.4. Catalyst Reusability
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. The Activated Carbon Characterization
3.2.2. KOH/AC Catalyst Preparation
3.2.3. Lipase Immobilization on AC
3.2.4. Vegetable Oil Characterization
3.2.5. Biodiesel Characterization
3.2.6. Transesterification of Vegetable Oil with KOH as a Catalyst
3.2.7. Transesterification of Vegetable Oil with Lipase as a Catalyst Supported on the Activated Carbon
4. Conclusions
- The use of AC as a carrier for KOH or lipase leads to carrying out the transesterification reaction more efficiently, in comparison to the homogeneous catalyst KOH;
- The use of AC to immobilize lipase allows an increase in the stability of EvTr, which allows for reuse in subsequent cycles up to the fifth cycle. Carrying out the enzymatic transesterification of vegetable oils in the presence of EvTr/AC results in no soap formation. Therefore, the steps of FAMEs and glycerol fraction purification in the industrial process of biodiesel production could be avoided.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dossat, V.; Combes, D.; Marty, A. Lipase-catalysed transesterification of high oleic sunflower oil. Enzym. Microb. Technol. 2002, 30, 90–94. [Google Scholar] [CrossRef]
- Encinar, J.M.; Nogales-Delgado, S.; Sánchez, N.; González, J.F. Biolubricants from rapeseed and castor oil transesterification by using titanium isopropoxide as a catalyst: Production and characterization. Catalysts 2020, 10, 366. [Google Scholar] [CrossRef] [Green Version]
- Esipovich, A.L.; Belousov, A.S.; Kanakov, E.A.; Mironova, V.Y.; Rogozhin, A.E.; Danov, S.M.; Vorotyntsev, A.V.; Makarov, D.A. Solvent effects in epoxidation of fatty acid methyl esters with hydrogen peroxide over TS-1 catalyst. Kinet. Catal. 2019, 60, 62–68. [Google Scholar] [CrossRef]
- Wenning, L.; Ejsing, C.S.; David, F.; Sprenger, R.R.; Nielsen, J.; Siewers, V. Increasing jojoba-like wax ester production in Saccharomyces cerevisiae by enhancing very long-chain, monounsaturated fatty acid synthesis. Microb. Cell Fact. 2019, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Salimon, J.; Salih, N.; Yousif, E. Industrial development and applications of plant oils and their biobased oleochemicals. Arab. J. Chem. 2012, 5, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Mekhilef, S.; Siga, S.; Saidur, R. A review on palm oil biodiesel as a source of renewable fuel. Renew. Sustain. Energy Rev. 2011, 15, 1937–1949. [Google Scholar] [CrossRef]
- Radzi, S.M.; Basri, M.; Salleh, A.B.; Ariff, A.; Mohammad, R.; Abdul Rahman, M.B.; Abdul Rahman, R.N.Z.R. Optimisation study of large-scale enzymatic synthesis of oleyl oleate, a liquid wax ester, by response surface methodology. J. Chem. Technol. Biotechnol. 2006, 81, 374–380. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S. An evaluation of multipurpose oil seed crop for industrial uses (Jatropha curcas L.): A review. Ind. Crop. Prod. 2008, 28, 1–10. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Kumar Sharma, P.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Lotero, E.; Liu, Y.; Lopez, D.E.; Suwannakarn, K.; Bruce, D.A.; Goodwin, J.G. Synthesis of biodiesel via acid catalysis. Ind. Eng. Chem. Res. 2005, 44, 5353–5363. [Google Scholar] [CrossRef]
- Taravus, S.; Temur, H.; Yartasi, A. Alkali-catalyzed biodiesel production from mixtures of sunflower oil and beef tallow. Energy Fuels 2009, 23, 4112–4115. [Google Scholar] [CrossRef]
- Terigar, B.G.; Balasubramanian, S.; Lima, M.; Boldor, D. Transesterification of Soybean and Rice Bran Oil with Ethanol in a Continuous-Flow Microwave-Assisted System: Yields, Quality, and Reaction Kinetics. Energy Fuels 2010, 24, 6609–6615. [Google Scholar] [CrossRef]
- Fadhil, A.B.; Saeed, L.I. Sulfonated tea waste: A low-cost adsorbent for purification of biodiesel. Int. J. Green Energy 2016, 13, 110–118. [Google Scholar] [CrossRef]
- Gog, A.; Roman, M.; Toşa, M.; Paizs, C.; Irimie, F.D. Biodiesel production using enzymatic transesterification—Current state and perspectives. Renew. Energy 2012, 39, 10–16. [Google Scholar] [CrossRef]
- Rathore, V.; Newalkar, B.L.; Badoni, R.P. Processing of vegetable oil for biofuel production through conventional and non-conventional routes. Energy Sustain. Dev. 2016, 31, 24–49. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Das, L.M. Process optimization for biodiesel production from Jatropha, Karanja and Polanga oils. Fuel 2009, 88, 1588–1594. [Google Scholar] [CrossRef]
- Sanli, H.; Canakci, M. Effects of different alcohol and catalyst usage on biodiesel. Energy Fuels 2008, 22, 2713–2719. [Google Scholar] [CrossRef]
- Meher, L.C.; Vidya Sagar, D.; Naik, S.N. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Talha, N.S.; Sulaiman, S. Overview of catalysts in biodiesel production. Arpn J. Eng. Appl. Sci. 2016, 11, 439–442. [Google Scholar]
- Maugard, T.; Tudella, J.; Legoy, M.D. Study of vitamin ester synthesis by lipase-catalyzed transesterification in organic media. Biotechnol. Prog. 2000, 16, 358–362. [Google Scholar] [CrossRef]
- Rafatullah, M.; Ahmad, T.; Ghazali, A.; Sulaiman, O.; Danish, M.; Hashim, R. Oil palm biomass as a precursor of activated carbons: A review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1117–1161. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S. Potential of palm kernel shell as activated carbon precursors through single stage activation technique for carbon dioxide adsorption. J. Clean. Prod. 2017, 168, 474–486. [Google Scholar] [CrossRef]
- Sajjadi, S.A.; Meknati, A.; Lima, E.C.; Dotto, G.L.; Mendoza-Castillo, D.I.; Anastopoulos, I.; Alakhras, F.; Unuabonah, E.I.; Singh, P.; Hosseini-Bandegharaei, A. A novel route for preparation of chemically activated carbon from pistachio wood for highly efficient Pb(II) sorption. J. Environ. Manag. 2019, 236, 34–44. [Google Scholar] [CrossRef]
- Dhawane, S.H.; Kumar, T.; Halder, G. Recent advancement and prospective of heterogeneous carbonaceous catalysts in chemical and enzymatic transformation of biodiesel. Energy Convers. Manag. 2018, 167, 176–202. [Google Scholar] [CrossRef]
- Gupta, V.K.; Nayak, A.; Bhushan, B.; Agarwal, S. A critical analysis on the efficiency of activated carbons from low-cost precursors for heavy metals remediation. Crit. Rev. Environ. Sci. Technol. 2014, 45, 613–668. [Google Scholar] [CrossRef]
- Halder, G.; Dhawane, S.; Barai, P.K.; Das, A. Optimizing chromium (VI) adsorption onto superheated steam activated granular carbon through response surface methodology and artificial neural network. Environ. Prog. Sustain. Energy 2015, 34, 638–647. [Google Scholar] [CrossRef]
- Vargas, A.M.M.; Garcia, C.A.; Reis, E.M.; Lenzi, E.; Costa, W.F.; Almeida, V.C. NaOH-activated carbon from flamboyant (Delonix regia) pods: Optimization of preparation conditions using central composite rotatable design. Chem. Eng. J. 2010, 162, 43–50. [Google Scholar] [CrossRef]
- Auer, E.; Freund, A.; Pietsch, J.; Tacke, T. Carbons as supports for industrial precious metal catalysts. Appl. Catal. A Gen. 1998, 173, 259–271. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R. The role of surface chemistry in catalysis with carbons. Catal. Today 2010, 150, 2–7. [Google Scholar] [CrossRef]
- Figueiredo, J.L. Functionalization of porous carbons for catalytic applications. J. Mater. Chem. A 2013, 1, 9351–9364. [Google Scholar] [CrossRef]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.W.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Wan, Z.; Hameed, B.H. Transesterification of palm oil to methyl ester on activated carbon supported calcium oxide catalyst. Bioresour. Technol. 2011, 102, 2659–2664. [Google Scholar] [CrossRef]
- Zu, Y.; Liu, G.; Wang, Z.; Shi, J.; Zhang, M.; Zhang, W.; Jia, M. CaO supported on porous carbon as highly efficient heterogeneous catalysts for transesterification of triacetin with methanol. Energy Fuels 2010, 24, 3810–3816. [Google Scholar] [CrossRef]
- Baroutian, S.; Aroua, M.K.; Raman, A.A.A.; Sulaiman, N.M.N. Potassium hydroxide catalyst supported on palm shell activated carbon for transesterification of palm oil. Fuel Process. Technol. 2010, 91, 1378–1385. [Google Scholar] [CrossRef]
- Dhawane, S.H.; Kumar, T.; Halder, G. Central composite design approach towards optimization of flamboyant pods derived steam activated carbon for its use as heterogeneous catalyst in transesterification of Hevea brasiliensis oil. Energy Convers. Manag. 2015, 100, 277–287. [Google Scholar] [CrossRef]
- Narowska, B.; Kułażyński, M.; Łukaszewicz, M.; Burchacka, E. Use of activated carbons as catalyst supports for biodiesel production. Renew. Energy 2019, 135, 176–185. [Google Scholar] [CrossRef]
- Giraldo, L.; Moreno-Piraján, J.C. Lipase supported on mesoporous materials as a catalyst in the synthesis of biodiesel from Persea americana mill oil. J. Mol. Catal. B Enzym. 2012, 77, 32–38. [Google Scholar] [CrossRef]
- Babel, S.; Arayawate, S.; Faedsura, E.; Sudrajat, H. Production of biodiesel from waste cooking oil using transesterification, with the KOH on carbon support from waste material and egg shell, as the catalyst. Environ. Nat. Resour. J. 2016, 14, 60–68. [Google Scholar]
- Fadhil, A.B.; Aziz, A.M.; Altamer, M.H. Potassium acetate supported on activated carbon for transesterification of new non-edible oil, bitter almond oil. Fuel 2016, 170, 130–140. [Google Scholar] [CrossRef]
- Ghosh, G.C.; Chakraborty, T.K.; Zaman, S.; Nahar, M.N.; Kabir, A.H.M.E. Removal of methyl orange dye from aqueous solution by a low-cost activated carbon prepared from mahagoni (Swietenia mahagoni) Bark. Pollution 2020, 6, 171–184. [Google Scholar]
- Timur, S.; Kantarli, I.C.; Onenc, S.; Yanik, J. Characterization and application of activated carbon produced from oak cups pulp. J. Anal. Appl. Pyrolysis 2010, 89, 129–136. [Google Scholar] [CrossRef]
- Anastopoulos, G.; Dodos, G.S.; Kalligeros, S.; Zannikos, F. Biodiesel production by ethanolysis of various vegetable oils using calcium ethoxide as a solid base catalyst. Int. J. Green Energy 2013, 10, 468–481. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Singh, B.; Korstad, J. High yield and conversion of biodiesel from a nonedible feedstock (Pongamia pinnata). J. Agric. Food Chem. 2010, 58, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Baroutian, S.; Aroua, M.K.; Raman, A.A.A.; Sulaiman, N.M.N. A packed bed membrane reactor for production of biodiesel using activated carbon supported catalyst. Bioresour. Technol. 2011, 102, 1095–1102. [Google Scholar] [CrossRef]
- Buasri, A.; Chaiyut, N.; Loryuenyong, V.; Rodklum, C.; Chaikwan, T.; Kumphan, N.; Jadee, K.; Klinklom, P. WittayaWittayarounayut Transesterification of waste frying oil for synthesizing biodiesel by KOH supported on coconut shell activated carbon in packed bed reactor. ScienceAsia 2012, 38, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Dhawane, S.H.; Kumar, T.; Halder, G. Biodiesel synthesis from Hevea brasiliensis oil employing carbon supported heterogeneous catalyst: Optimization by Taguchi method. Renew. Energy 2016, 89, 506–514. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kang, B.-S.; Kim, M.-J.; Park, Y.M.; Kim, D.-K.; Lee, J.-S.; Lee, K.-Y. Transesterification of vegetable oil to biodiesel using heterogeneous base catalyst. Catal. Today 2004, 93–95, 315–320. [Google Scholar] [CrossRef]
- Liu, X.; Piao, X.; Wang, Y.; Zhu, S.; He, H. Calcium methoxide as a solid base catalyst for the transesterification of soybean oil to biodiesel with methanol. Fuel 2008, 87, 1076–1082. [Google Scholar] [CrossRef]
- Alam, M.Z.; Asih, D.R.; Salleh, M.N. Immobilization of lipase enzyme by low cost material: A statistical approach. Int. J. Biol. Vet. Agric. Food Eng. 2014, 8, 522–525. [Google Scholar]
- Quirós, M.; García, A.B.; Montes-Morán, M.A. Influence of the support surface properties on the protein loading and activity of lipase/mesoporous carbon biocatalysts. Carbon N. Y. 2011, 49, 406–415. [Google Scholar] [CrossRef]
- Vicente, G.; Martínez, M.; Aracil, J. Integrated biodiesel production: A comparison of different homogeneous catalysts systems. Bioresour. Technol. 2004, 92, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Vinu, A.; Miyahara, M.; Mori, T.; Ariga, K. Carbon nanocage: A large-pore cage-type mesoporous carbon material as an adsorbent for biomolecules. J. Porous Mater. 2006, 13, 379–383. [Google Scholar] [CrossRef]
- Dhawane, S.H.; Kumar, T.; Halder, G. Insight into biodiesel synthesis using biocatalyst designed through lipase immobilization onto waste derived microporous carbonaceous support. Process. Saf. Environ. Prot. 2019, 124, 231–239. [Google Scholar] [CrossRef]
- Naranjo, J.C.; Cordoba, A.; Giraldo, L.; Garcia, V.S.; Moreno-Pirajan, J.C. Lipase supported on granular activated carbon and activated carbon cloth as a catalyst in the synthesis of biodiesel fuel. J. Mol. Catal. B Enzym. 2010, 66, 166–171. [Google Scholar] [CrossRef]
- Lopes, J.C.A.; Boros, L.; Kráhenbúhl, M.A.; Meirelles, A.J.A.; Daridon, J.L.; Pauly, J.; Marrucho, I.M.; Coutinho, J.A.P. Prediction of cloud points of biodiesel. Energy Fuels 2008, 22, 747–752. [Google Scholar] [CrossRef]
- Chuah, L.F.; Yusup, S.; Aziz, A.R.A.; Klemeš, J.J.; Bokhari, A.; Abdullah, M.Z. Influence of fatty acids content in non-edible oil for biodiesel properties. Clean Technol. Environ. Policy 2016, 18, 473–482. [Google Scholar] [CrossRef]
- Dorado, M.P.; Ballesteros, E.; Mittelbach, M.; López, F.J. Kinetic parameters affecting the alkali-catalyzed transesterification process of used olive oil. Energy Fuels 2004, 18, 1457–1462. [Google Scholar] [CrossRef]
- Candeia, R.A.; Silva, M.C.D.; Carcalho Filho, J.R.; Brasilino, M.G.A.; Bicudo, T.C.; Sanos, I.M.G.; Souza, A.G. Influence of soybean biodiesel content on basic properties of biodiesel-diesel blends. Fuel 2009, 88, 738–743. [Google Scholar] [CrossRef]
- Samukawa, T.; Kaieda, M.; Matsumoto, T.; Ban, K.; Kondo, A.; Shimada, Y.; Noda, H.; Fukuda, H. Pretreatment of immobilized Candida antarctica lipase for biodiesel fuel production from plant oil. J. Biosci Bioeng 2000, 90, 180–183. [Google Scholar] [CrossRef]
- Brito, M.J.P.; Veloso, C.M.; Bonomo, R.C.F.; Fontan, R.d.C.I.; Santos, L.S.; Monteiro, K.A. Activated carbons preparation from yellow mombin fruit stones for lipase immobilization. Fuel Process. Technol. 2017, 156, 421–428. [Google Scholar] [CrossRef]
- Tańska, M.; Rotkiewicz, D.; Urbalewicz, A. Characteristics of post-frying fats in terms of usefulness for biodiesel production. Nauk. Przyr. Technol. 2012, 6, 1–12. [Google Scholar]




| FAMEs | Amount of Catalyst (wt.%) | Reaction Time (h) | Density at 15 °C (kg/m3) | Viscosity at 40 °C (mm2/s) | CFPP (°C) |
|---|---|---|---|---|---|
| ROME | 0.5 | 1 | 882 | 5.1 | −1 |
| 0.5 | 4 | 882 | 4.8 | −4 | |
| 1.5 | 1 | 882 | 4.4 | −10 | |
| 1.5 | 4 | 880 | 4.4 | −5 | |
| SOME | 0.5 | 1 | 885 | 4.5 | 3 |
| 0.5 | 4 | 883 | 4.2 | 2 | |
| 1.5 | 1 | 883 | 4.2 | 0 | |
| 1.5 | 4 | 882 | 4.3 | 3 | |
| COME | 0.5 | 1 | 885 | 4.2 | 1 |
| 0.5 | 4 | 886 | 4.5 | 0 | |
| 1.5 | 1 | 883 | 4.5 | −3 | |
| 1.5 | 4 | 883 | 4.3 | −1 | |
| OOME | 0.5 | 1 | 877 | 4.9 | 0 |
| 0.5 | 4 | 877 | 4.6 | 1 | |
| 1.5 | 1 | 887 | 4.6 | −2 | |
| 1.5 | 4 | 876 | 4.6 | 1 |
| FAME | Number of Reuse | Amount of Catalyst (wt.%) | Reaction Time (h) | Density at 15 °C (kg/m3) | Viscosity at 40 °C (mm2/s) | CFPP (°C) |
|---|---|---|---|---|---|---|
| ROME | I | 0.5 | 1 | 889 | 5.8 | 4 |
| II | 0.5 | 1 | 907 | 14.5 | 14 | |
| I | 0.5 | 4 | 887 | 5.0 | 1 | |
| II | 0.5 | 4 | 903 | 11.7 | 15 | |
| I | 1.5 | 1 | 885 | 5.0 | −3 | |
| II | 1.5 | 1 | 892 | 6.4 | 4 | |
| I | 1.5 | 4 | 886 | 4.6 | −5 | |
| II | 1.5 | 4 | 890 | 6.4 | −2 | |
| SOME | I | 0.5 | 1 | 890 | 5.5 | 4 |
| II | 0.5 | 1 | 916 | 15.8 | 16 | |
| I | 0.5 | 4 | 888 | 4.8 | −5 | |
| II | 0.5 | 4 | 910 | 12.3 | 16 | |
| I | 1.5 | 1 | 886 | 4.6 | −4 | |
| II | 1.5 | 1 | 895 | 6.3 | 5 | |
| I | 1.5 | 4 | 885 | 4.4 | −6 | |
| II | 1.5 | 4 | 897 | 6.9 | −1 | |
| COME | I | 0.5 | 1 | 895 | 6.3 | 6 |
| II | 0.5 | 1 | 909 | 14.3 | 15 | |
| I | 0.5 | 4 | 888 | 5.1 | 1 | |
| II | 0.5 | 4 | 905 | 11.7 | 14 | |
| I | 1.5 | 1 | 886 | 4.6 | −5 | |
| II | 1.5 | 1 | 894 | 6.3 | 4 | |
| I | 1.5 | 4 | 886 | 4.3 | −6 | |
| II | 1.5 | 4 | 890 | 6.0 | −8 | |
| OOME | I | 0.5 | 1 | 887 | 7.5 | 7 |
| II | 0.5 | 1 | 915 | 32.3 | 21 | |
| I | 0.5 | 4 | 885 | 6.0 | 4 | |
| II | 0.5 | 4 | 911 | 29.6 | 20 | |
| I | 1.5 | 1 | 880 | 5.0 | −2 | |
| II | 1.5 | 1 | 896 | 9.2 | 11 | |
| I | 1.5 | 4 | 880 | 4.7 | −6 | |
| II | 1.5 | 4 | 890 | 8.6 | 8 |
| FAME | Cycle Number | Density at 15 °C (kg/m3) | Viscosity at 40 °C (mm2/s) | CFPP (°C) |
|---|---|---|---|---|
| ROME | I | 883 | 4.8 | −13 |
| II | 883 | 4.7 | −13 | |
| III | 883 | 4.7 | −12 | |
| IV | 887 | 5.5 | −7 | |
| V | 889 | 6.4 | −1 | |
| SOME | I | 895 | 7.3 | −6 |
| II | 888 | 6.3 | −8 | |
| III | 893 | 7.3 | −7 | |
| COME | I | 885 | 4.7 | −12 |
| II | 885 | 4.7 | −5 | |
| III | 886 | 4.7 | −4 | |
| IV | 891 | 4.1 | −4 | |
| V | 893 | 7.3 | −1 | |
| OOME | I | 892 | 9.7 | −3 |
| II | 887 | 6.5 | −5 | |
| III | 895 | 9.8 | −3 |
| No. | Property | Unit | Vegetable Oil | ||||
|---|---|---|---|---|---|---|---|
| Rapeseed Oil | Sunflower Oil | Olive Oil | Corn Oil | Waste Corn Oil [36] | |||
| 1 | Free fatty acid content | wt.% | 0.04 | 0.05 | 0.37 | 0.04 | 14.11 |
| 2 | Water content | wt.% | 0.08 | 0.05 | 0.04 | 0.04 | 0.07 |
| 3 | Fatty acid composition | wt.% | |||||
| 4 | Myristic; C 14:0 | wt.% | 0.3 | 0.3 | 0.3 | 0.4 | 0.1 |
| Palmitic; C 16:0 | 4.2 | 7.4 | 14.0 | 10.7 | 10.1 | ||
| Palmitoleic; C 16:1 | 0.3 | 0.3 | 1.8 | 0.3 | 2.0 | ||
| Stearic; C 18:0 | 1.6 | 3.8 | 2.7 | 1.9 | 1.8 | ||
| Oleic; C 18:1 | 64.1 | 29.4 | 69.0 | 30.9 | 28.6 | ||
| Linoleic; C 18:2 | 18.1 | 56.9 | 10.7 | 54.7 | 55.6 | ||
| Linolenic; C 18:3 | 8.5 | 0.0 | 0.0 | 0.0 | 1.1 | ||
| Arachidic; C 20:0 | 0.7 | 0.3 | 0.5 | 0.6 | 0.4 | ||
| Gadoleic; C 20:1 | 1.3 | 0.1 | 0.3 | 0.2 | 0.3 | ||
| Behenic; C 22:0 | 0.4 | 0.9 | 0.1 | 0.2 | 0.0 | ||
| Erucic; C 22:1 | 0.2 | 0.2 | 0.0 | 0.0 | 0.0 | ||
| Lignoceric; C 24:0 | 0.1 | 0.3 | 0.1 | 0.1 | 0.0 | ||
| Nervonic; C 24:1 | 0.2 | 0.1 | 0.5 | 0.0 | 0.0 | ||
| 5 | Density at 15 °C | kg/m3 | 924 | 920 | 920 | 924 | 925 |
| 6 | Viscosity at 40 °C | mm2/s | 32.0 | 34.0 | 38.2 | 33.7 | 28.8 |
| 7 | CFPP | °C | 21 | 19 | 20 | 19 | 20 |
| Property | Unit | FAME | EN 14214 | ||||
|---|---|---|---|---|---|---|---|
| ROME I Use | ROME II Use | COME I Use | COME II Use | COMEw I Use | |||
| FAME content | wt.% | 96.3 | 83.9 | 91.9 | 82.4 | 98.5 | ≥96.5 |
| Monoglyceride content | wt.% | 0.67 | 0.99 | 0.56 | 1.16 | 0.38 1 | <0.70 |
| Diglyceride content | wt.% | 0.55 | 3.62 | 0.30 | 3.03 | 0.24 1 | <0.20 |
| Triglyceride content | wt.% | 1.64 | 8.81 | 0.76 | 7.10 | 0.87 1 | <0.20 |
| Free glycerol | wt.% | 0.004 | 0.004 | 0.005 | 0.006 | 0.010 1 | <0.02 |
| Total glycerol | wt.% | 0.422 | 1.691 | 0.271 | 1.476 | 0.230 1 | <0.25 |
| Acid value | mgKOH/g | 0.16 | 0.19 | 0.15 | 0.15 | 0.08 1 | <0.50 |
| Methanol value | wt.% | 0.002 | 0 | 0.001 | 0 | 0.70 1 | <0.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narowska, B.E.; Kułażyński, M.; Łukaszewicz, M. Application of Activated Carbon to Obtain Biodiesel from Vegetable Oils. Catalysts 2020, 10, 1049. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091049
Narowska BE, Kułażyński M, Łukaszewicz M. Application of Activated Carbon to Obtain Biodiesel from Vegetable Oils. Catalysts. 2020; 10(9):1049. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091049
Chicago/Turabian StyleNarowska, Beata Edyta, Marek Kułażyński, and Marcin Łukaszewicz. 2020. "Application of Activated Carbon to Obtain Biodiesel from Vegetable Oils" Catalysts 10, no. 9: 1049. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091049