Direct Synthesis of Dimethyl Ether from Syngas on Bifunctional Hybrid Catalysts Based on Supported H3PW12O40 and Cu-ZnO(Al): Effect of Heteropolyacid Loading on Hybrid Structure and Catalytic Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure and Acidity of xHPW/Ti Acid Catalysts
2.2. Structure and Acidity of CZA-xHPW/Ti Bifunctional Hybrid Catalysts
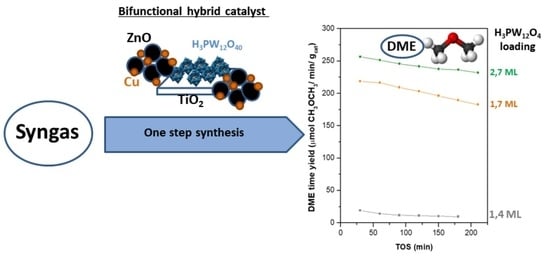
2.3. Activity in Direct Synthesis of DME from Syngas
3. Materials and Methods
3.1. Catalysts Preparation
3.1.1. Cu-ZnO(Al) (CZA) Methanol Synthesis Catalyst
3.1.2. H3PW12O40/TiO2 Acid Catalysts
3.1.3. Bifunctional CZA-xHPW/Ti Hybrid Catalysts
3.2. Physicochemical Characterization
3.3. DME Synthesis from Syngas Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arcoumanis, C.; Bae, C.; Crookes, R.; Kinoshita, E. The potential of di-methyl ether (DME) as an alternative fuel for compression-ignition engines: A review. Fuel 2008, 87, 1014–1030. [Google Scholar] [CrossRef]
- Sun, J.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Catalysis chemistry of dimethyl ether synthesis. ACS Catal. 2014, 4, 3346–3356. [Google Scholar] [CrossRef]
- Semelsberger, T.A.; Borup, R.L.; Greene, H.L. Dimethyl ether (DME) as an alternative fuel. J. Power Sources 2006, 156, 497–511. [Google Scholar] [CrossRef]
- Jung, J.W.; Lee, Y.J.; Um, S.H.; Yoo, P.J.; Lee, D.H.; Jun, K.-W.; Bae, J.W. Effect of copper surface area and acidic sites to intrinsic catalytic activity for dimethyl ether synthesis from biomass-derived syngas. Appl. Catal. B Environ. 2012, 126, 1–8. [Google Scholar] [CrossRef]
- Bonura, G.; Cordaro, M.; Spadaro, L.; Cannilla, C.; Arena, F.; Frusteri, F. Hybrid Cu–ZnO–ZrO2/h-ZSM5 system for the direct synthesis of DME by CO2 hydrogenation. Appl. Catal. B Environ. 2013, 140–141, 16–24. [Google Scholar] [CrossRef]
- Behrens, M.; Studt, F.; Kasatkin, I.; Kühl, S.; Hävecker, M.; Abild-Pedersen, F.; Zander, S.; Girgsdies, F.; Kurr, P.; Kniep, B.-L.; et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 2012, 336, 893–897. [Google Scholar] [CrossRef]
- Grunwaldt, J.D.; Molenbroek, A.M.; Topsøe, N.Y.; Topsøe, H.; Clausen, B.S. In situ investigations of structural changes in Cu/ZnO catalysts. J. Catal. 2000, 194, 452–460. [Google Scholar] [CrossRef]
- Kanai, Y.; Watanabe, T.; Fujitani, T.; Saito, M.; Nakamura, J.; Uchijima, T. Evidence for the migration of ZnOx in a Cu/ZnO methanol synthesis catalyst. Catal. Lett. 1994, 27, 67–78. [Google Scholar] [CrossRef]
- Cai, M.; Palčić, A.; Subramanian, V.; Moldovan, S.; Ersen, O.; Valtchev, V.; Ordomsky, V.V.; Khodakov, A.Y. Direct dimethyl ether synthesis from syngas on copper–zeolite hybrid catalysts with a wide range of zeolite particle sizes. J. Catal. 2016, 338, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Xie, Q.; Chen, P.; Peng, P.; Liu, S.; Peng, P.; Zhang, B.; Cheng, Y.; Wan, Y.; Liu, Y.; Ruan, R. Single-step synthesis of DME from syngas on cuznal–zeolite bifunctional catalysts: The influence of zeolite type. RSC Adv. 2015, 5, 26301–26307. [Google Scholar] [CrossRef]
- Takeguchi, T.; Yanagisawa, K.-I.; Inui, T.; Inoue, M. Effect of the property of solid acid upon syngas-to-dimethyl ether conversion on the hybrid catalysts composed of Cu–Zn–Ga and solid acids. Appl. Catal. A Gen. 2000, 192, 201–209. [Google Scholar] [CrossRef]
- Abu-Dahrieh, J.; Rooney, D.; Goguet, A.; Saih, Y. Activity and deactivation studies for direct dimethyl ether synthesis using CuO–ZnO–Al2O3 with NH4ZSM-5, HZSM-5 or γ-Al2O3. Chem. Eng. J. 2012, 203, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Kozhevnikov, I.V. Sustainable heterogeneous acid catalysis by heteropoly acids. J. Mol. Catal. A Chem. 2007, 262, 86–92. [Google Scholar] [CrossRef]
- Janik, M.J.; Campbell, K.A.; Bardin, B.B.; Davis, R.J.; Neurock, M. A computational and experimental study of anhydrous phosphotungstic acid and its interaction with water molecules. Appl. Catal. A Gen. 2003, 256, 51–68. [Google Scholar] [CrossRef]
- Micek-Ilnicka, A. The role of water in the catalysis on solid heteropolyacids. J. Mol. Catal. A Chem. 2009, 308, 1–14. [Google Scholar] [CrossRef]
- Schnee, J.; Gaigneaux, E.M. Elucidating and exploiting the chemistry of keggin heteropolyacids in the methanol-to-DME conversion: Enabling the bulk reaction thanks to operando raman. Catal. Sci. Technol. 2017, 7, 817–830. [Google Scholar] [CrossRef]
- Uchida, S.; Inumaru, K.; Misono, M. States and dynamic behavior of protons and water molecules in H3PW12O40 pseudoliquid phase analyzed by solid-state MAS NMR. J. Phys. Chem. B 2000, 104, 8108–8115. [Google Scholar] [CrossRef]
- Schnee, J.; Eggermont, A.; Gaigneaux, E.M. Boron nitride: A support for highly active heteropolyacids in the methanol-to-DME reaction. ACS Catal. 2017, 7, 4011–4017. [Google Scholar] [CrossRef]
- Ladera, R.M.; Ojeda, M.; Fierro, J.L.G.; Rojas, S. TiO2-supported heteropoly acid catalysts for dehydration of methanol to dimethyl ether: Relevance of dispersion and support interaction. Catal. Sci. Technol. 2015, 5, 484–491. [Google Scholar] [CrossRef]
- García-López, E.I.; Marcì, G.; Krivtsov, I.; Casado Espina, J.; Liotta, L.F.; Serrano, A. Local structure of supported keggin and wells–dawson heteropolyacids and its influence on the catalytic activity. J. Phys. Chem. C 2019, 123, 19513–19527. [Google Scholar] [CrossRef]
- Alharbi, W.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Dehydration of methanol to dimethyl ether over heteropoly acid catalysts: The relationship between reaction rate and catalyst acid strength. ACS Catal. 2015, 5, 7186–7193. [Google Scholar] [CrossRef]
- Newman, A.D.; Brown, D.R.; Siril, P.; Lee, A.F.; Wilson, K. Structural studies of high dispersion H3PW12O40/SiO2 solid acid catalysts. Phys. Chem. Chem. Phys. 2006, 8, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Moradi, G.R.; Nosrati, S.; Yaripor, F. Effect of the hybrid catalysts preparation method upon direct synthesis of dimethyl ether from synthesis gas. Catal. Commun. 2007, 8, 598–606. [Google Scholar] [CrossRef]
- García-Trenco, A.; Vidal-Moya, A.; Martínez, A. Study of the interaction between components in hybrid CuZnAl/HZSM-5 catalysts and its impact in the syngas-to-DME reaction. Catal. Today 2012, 179, 43–51. [Google Scholar] [CrossRef]
- Marosi, L.; Escalona Platero, E.; Cifre, J.; Otero Areán, C. Thermal dehydration of H3+xPVxM12−xO40·yH2O keggin type heteropolyacids; formation, thermal stability and structure of the anhydrous acids H3PM12O40, of the corresponding anhydrides PM12O38.5 and of a novel trihydrate H3PM12O40·3H2O. J. Mater. Chem. 2000, 10, 1949–1955. [Google Scholar] [CrossRef]
- Mioč, U.B.; Dimitrijević, R.Ž.; Davidović, M.; Nedić, Z.P.; Mitrović, M.M.; Colomban, P. Thermally induced phase transformations of 12-tungstophosphoric acid 29-hydrate: Synthesis and characterization of PW8O26-type bronzes. J. Mater. Sci. 1994, 29, 3705–3718. [Google Scholar] [CrossRef]
- Gallardo, R.M.L. Desarrollo de de Wolframio Altamente Activos y Selectivos para la Síntesis de Dimetiléter. Efecto de la Estructura y de la Interacción con el Soporte. Departamento de Química-Física Aplicada, Universidad Autónoma de Madrid. 2013. Available online: https://digital.csic.es/bitstream/10261/80583/1/Ladera%20Gallardo,%20R.M._Tesis_2013.pdf (accessed on 15 September 2020).
- Alsalme, A.M.; Wiper, P.V.; Khimyak, Y.Z.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Solid acid catalysts based on H3PW12O40 heteropoly acid: Acid and catalytic properties at a gas–solid interface. J. Catal. 2010, 276, 181–189. [Google Scholar] [CrossRef]
- Da Silva, M.J.; de Oliveira, C.M. Catalysis by keggin heteropolyacid salts. Curr. Catal. 2018, 7, 26–34. [Google Scholar] [CrossRef]
- Bielański, A.; Lubańska, A. Ftir investigation on wells–dawson and keggin type heteropolyacids: Dehydration and ethanol sorption. J. Mol. Catal. A Chem. 2004, 224, 179–187. [Google Scholar] [CrossRef]
- Jeantelot, G.; Ould-Chikh, S.; Sofack-Kreutzer, J.; Abou-Hamad, E.; Anjum, D.H.; Lopatin, S.; Harb, M.; Cavallo, L.; Basset, J.-M. Morphology control of anatase TiO2 for well-defined surface chemistry. Phys. Chem. Chem. Phys. 2018, 20, 14362–14373. [Google Scholar] [CrossRef] [Green Version]
- Agmon, N. The acid test for water structure. Nat. Chem. 2016, 8, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Kulig, W.; Agmon, N. A ‘clusters-in-liquid’ method for calculating infrared spectra identifies the proton-transfer mode in acidic aqueous solutions. Nat. Chem. 2013, 5, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Agmon, N. Reinvestigation of the infrared spectrum of the gas-phase protonated water tetramer. J. Phys. Chem. A 2017, 121, 3056–3070. [Google Scholar] [CrossRef]
- Bielański, A.; Małecka, A.; Kybelkova, L. Infrared study of the thermal decomposition of heteropolyacids of the series H3+xPMo12−xVxO40. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens./Phases 1989, 85, 2847–2856. [Google Scholar] [CrossRef]
- Hardcastle, F.D. Raman spectroscopy of titania (TiO2) nanotubular water-splitting catalysts. J. Ark. Acad. Sci. 2011, 65, 43–48. [Google Scholar]
- Lagopati, N.; Tsilibary, E.P.; Falaras, P.; Papazafiri, P.; Pavlatou, E.A.; Kotsopoulou, E.; Kitsiou, P. Effect of nanostructured TiO2 crystal phase on photoinduced apoptosis of breast cancer epithelial cells. Int. J. Nanomed. 2014, 9, 3219–3230. [Google Scholar] [CrossRef] [Green Version]
- Jagadeeswaraiah, K.; Kumar, C.R.; Prasad, P.S.S.; Lingaiah, N. Incorporation of Zn2+ ions into the secondary structure of heteropoly tungstate: Catalytic efficiency for synthesis of glycerol carbonate from glycerol and urea. Catal. Sci. Technol. 2014, 4, 2969–2977. [Google Scholar] [CrossRef]
- Chai, S.-H.; Wang, H.-P.; Liang, Y.; Xu, B.-Q. Sustainable production of acrolein: Gas-phase dehydration of glycerol over 12-tungstophosphoric acid supported on ZrO2 and SiO2. Green Chem. 2008, 10, 1087–1093. [Google Scholar] [CrossRef]
- Caliman, E.; Dias, J.A.; Dias, S.C.L.; Prado, A.G.S. Solvent effect on the preparation of H3PW12O40 supported on alumina. Catal. Today 2005, 107–108, 816–825. [Google Scholar] [CrossRef]
- Shi, W.; Zhao, J.; Yuan, X.; Wang, S.; Wang, X.; Huo, M. Effects of brønsted and lewis acidities on catalytic activity of heteropolyacids in transesterification and esterification reactions. Chem. Eng. Technol. 2012, 35, 347–352. [Google Scholar] [CrossRef]
- García-López, E.I.; Marcì, G.; Pomilla, F.R.; Kirpsza, A.; Micek-Ilnicka, A.; Palmisano, L. Supported H3PW12O40 for 2-propanol (photo-assisted) catalytic dehydration in gas-solid regime: The role of the support and of the pseudo-liquid phase in the (photo)activity. Appl. Catal. B Environ. 2016, 189, 252–265. [Google Scholar] [CrossRef]
- Caliman, E.; Dias, J.A.; Dias, S.C.L.; Garcia, F.A.C.; de Macedo, J.L.; Almeida, L.S. Preparation and characterization of H3PW12O40 supported on niobia. Microporous Mesoporous Mater. 2010, 132, 103–111. [Google Scholar] [CrossRef]
- Nakabayashi, H.; Kakuta, N.; Ueno, A. Strong acid sites created on small–sized anatase. Bull. Chem. Soc. Jpn. 1991, 64, 2428–2432. [Google Scholar] [CrossRef] [Green Version]
- Essayem, N.; Frety, R.; Coudurier, G.; Vedrine, J.C. Ammonia adsorption–desorption over the strong solid acid catalyst H3PW12O40 and its Cs+ and NH4+ salts comparison with sulfated zirconia. J. Chem. Soc. Faraday Trans. 1997, 93, 3243–3248. [Google Scholar] [CrossRef]
- Southward, B.W.L.; Vaughan, J.S.; Oconnor, C.T. Infrared and thermal analysis studies of heteropoly acids. J. Catal. 1995, 153, 293–303. [Google Scholar] [CrossRef]
- Grinenval, E.; Rozanska, X.; Baudouin, A.; Berrier, E.; Delbecq, F.; Sautet, P.; Basset, J.-M.; Lefebvre, F. Controlled interactions between anhydrous keggin-type heteropolyacids and silica support: Preparation and characterization of well-defined silica-supported polyoxometalate species. J. Phys. Chem. C 2010, 114, 19024–19034. [Google Scholar] [CrossRef]
- Agosta, L.; Brandt, E.G.; Lyubartsev, A.P. Diffusion and reaction pathways of water near fully hydrated TiO2 surfaces from ab initio molecular dynamics. J. Chem. Phys. 2017, 147, 024704. [Google Scholar] [CrossRef] [Green Version]
- Melián-Cabrera, I.; López Granados, M.; Fierro, J.L.G. Structural reversibility of a ternary CuO-ZnO-Al2O3 ex hydrotalcite-containing material during wet pd impregnation. Catal. Lett. 2002, 84, 153–161. [Google Scholar] [CrossRef]
- Guil-López, R.; Mota, N.; Llorente, J.; Millán, E.; Pawelec, B.; García, R.; Fierro, J.L.G.; Navarro, R.M. Structure and activity of Cu/ZnO catalysts co-modified with aluminium and gallium for methanol synthesis. Catal. Today 2019. [Google Scholar] [CrossRef]
- Breen, J.P.; Ross, J.R.H. Methanol reforming for fuel-cell applications: Development of zirconia-containing Cu–Zn–Al catalysts. Catal. Today 1999, 51, 521–533. [Google Scholar] [CrossRef]
- Kuld, S.; Conradsen, C.; Moses, P.G.; Chorkendorff, I.; Sehested, J. Quantification of zinc atoms in a surface alloy on copper in an industrial-type methanol synthesis catalyst. Angew. Chem. Int. Ed. 2014, 53, 5941–5945. [Google Scholar] [CrossRef]
- García-Trenco, A.; Martínez, A. Direct synthesis of dme from syngas on hybrid CuZnAl/ZSM-5 catalysts: New insights into the role of zeolite acidity. Appl. Catal. A Gen. 2012, 411–412, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Toseland, B.; Underwood, R. Studies in surface science and catalysis. In Catalyst Deactivation; Bartholomew, G.A.F.C.H., Ed.; Elsevier Science: Amsterdam, The Netherlands, 1997; pp. 175–182. [Google Scholar]
- Ordomsky, V.V.; Cai, M.; Sushkevich, V.; Moldovan, S.; Ersen, O.; Lancelot, C.; Valtchev, V.; Khodakov, A.Y. The role of external acid sites of ZSM-5 in deactivation of hybrid CuZnAl/ZSM-5 catalyst for direct dimethyl ether synthesis from syngas. Appl. Catal. A Gen. 2014, 486, 266–275. [Google Scholar] [CrossRef]
- Sun, J.; Yang, G.; Ma, Q.; Ooki, I.; Taguchi, A.; Abe, T.; Xie, Q.; Yoneyama, Y.; Tsubaki, N. Fabrication of active Cu–Zn nanoalloys on H-ZSM5 zeolite for enhanced dimethyl ether synthesis via syngas. J. Mater. Chem. A 2014, 2, 8637–8643. [Google Scholar] [CrossRef]
- Flores, J.H.; Pais da Silva, M.I. Acid properties of the hybrid catalyst CuO–ZnO or CuO–ZnO–Al2O3/h-ferrierite: An infrared study. Coll. Surf. A Physicochem. Eng. Asp. 2008, 322, 113–123. [Google Scholar] [CrossRef]
- Yamamoto, H. From designer lewis acid to designer brønsted acid towards more reactive and selective acid catalysis. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2008, 84, 134–146. [Google Scholar] [CrossRef] [Green Version]
- Tao, M.; Xue, L.; Sun, Z.; Wang, S.; Wang, X.; Shi, J. Tailoring the synergistic bronsted-lewis acidic effects in heteropolyacid catalysts: Applied in esterification and transesterification reactions. Sci. Rep. 2015, 5, 13764. [Google Scholar] [CrossRef]
- Karcz, R.; Niemiec, P.; Pamin, K.; Połtowicz, J.; Kryściak-Czerwenka, J.; Napruszewska, B.D.; Michalik-Zym, A.; Witko, M.; Tokarz-Sobieraj, R.; Serwicka, E.M. Effect of cobalt location in keggin-type heteropoly catalysts on aerobic oxidation of cyclooctane: Experimental and theoretical study. Appl. Catal. A Gen. 2017, 542, 317–326. [Google Scholar] [CrossRef]
- Fyfe, C.A.; Kokotailo, G.T.; Graham, J.D.; Browning, C.; Gobbi, G.C.; Hyland, M.; Kennedy, G.J.; DeSchutter, C.T. Demonstration of contact induced ion exchange in zeolites. J. Am. Chem. Soc. 1986, 108, 522–523. [Google Scholar] [CrossRef]
- Borbely, G.; Beyer, H.K.; Radics, L.; Sandor, P.; Karge, H.G. Solid-state ion exchange in zeolites: Part iv. Evidence for contact-induced ion exchange between hydrated nay zeolite and metal chlorides. Zeolites 1989, 9, 428–431. [Google Scholar] [CrossRef]
- Schumann, J.; Lunkenbein, T.; Tarasov, A.; Thomas, N.; Schlögl, R.; Behrens, M. Synthesis and characterisation of a highly active Cu/ZnO: Al catalyst. ChemCatChem 2014, 6, 2889–2897. [Google Scholar] [CrossRef]
- Chang, C.D. Hydrocarbons from methanol. Catal. Rev. 1983, 25, 1–118. [Google Scholar] [CrossRef]
- Dadgar, F.; Myrstad, R.; Pfeifer, P.; Holmen, A.; Venvik, H.J. Direct dimethyl ether synthesis from synthesis gas: The influence of methanol dehydration on methanol synthesis reaction. Catal. Today 2016, 270, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Baltes, C.; Vukojević, S.; Schüth, F. Correlations between synthesis, precursor, and catalyst structure and activity of a large set of CuO/ZnO/Al2O3 catalysts for methanol synthesis. J. Catal. 2008, 258, 334–344. [Google Scholar] [CrossRef]
- Vedage, G.A.; Pitchai, R.; Herman, R.G.; Klier, K. Water promotion and identification of intermediates in methanol synthesis. In Proceedings of the 8th International Congress on Catalysis, Berlin, Germany, 2–6 July 1984; Verlag Chemie: Frankfurt, Germany; International Union of Pure Applied Chemistry: Research Triangle Park, NC, USA, 1984; pp. 47–59. [Google Scholar]
- Prašnikar, A.; Pavlišič, A.; Ruiz-Zepeda, F.; Kovač, J.; Likozar, B. Mechanisms of copper-based catalyst deactivation during CO2 reduction to methanol. Ind. Eng. Chem. Res. 2019, 58, 13021–13029. [Google Scholar] [CrossRef] [Green Version]
- Shikata, S.; Okuhara, T.; Misono, M. Catalysis by hetropoly compounds. Part XXVI. Gas phase synthesis of methyl tert-butyl ether over heteropolyacids. J. Mol. Catal. A Chem. 1995, 100, 49–59. [Google Scholar] [CrossRef]
- Rykova, A.I.; Burkat, T.M.; Pak, V.N. Hydrate and alcoholate forms of phosphomolybdic heteropolyacid and their formation under conditions of isothermal sorption of water, methanol, and ethanol vapors. Rus. J. Gen. Chem. 2003, 73, 697–700. [Google Scholar] [CrossRef]
- Dadgar, F.; Myrstad, R.; Pfeifer, P.; Holmen, A.; Venvik, H.J. Catalyst deactivation during one-step dimethyl ether synthesis from synthesis gas. Catal. Lett. 2017, 147, 865–879. [Google Scholar] [CrossRef]
- Schnee, J.; Gaigneaux, E.M. Lifetime of the H3PW12O40 heteropolyacid in the methanol-to-DME process: A question of pre-treatment. Appl. Catal. A Gen. 2017, 538, 174–180. [Google Scholar] [CrossRef]
- Mota, N.; Guil-Lopez, R.; Pawelec, B.G.; Fierro, J.L.G.; Navarro, R.M. Highly active Cu/ZnO–Al catalyst for methanol synthesis: Effect of aging on its structure and activity. RSC Adv. 2018, 8, 20619–20629. [Google Scholar] [CrossRef] [Green Version]
- Kaba, M.S.; Song, I.K.; Duncan, D.C.; Hill, C.L.; Barteau, M.A. Molecular shapes, orientation, and packing of polyoxometalate arrays imaged by scanning tunneling microscopy. Inorg. Chem. 1998, 37, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Park, M.J.; Kim, S.J.; Joo, O.-S.; Jung, K.-D. Dme synthesis from synthesis gas on the admixed catalysts of Cu/ZnO/Al2O3 and ZSM-5. Appl. Catal. A Gen. 2004, 264, 37–41. [Google Scholar] [CrossRef]
- Mao, D.; Yang, W.; Xia, J.; Zhang, B.; Song, Q.; Chen, Q. Highly effective hybrid catalyst for the direct synthesis of dimethyl ether from syngas with magnesium oxide-modified HZSM-5 as a dehydration component. J. Catal. 2005, 230, 140–149. [Google Scholar] [CrossRef]
- Bond, G.C.; Namijo, S.N. An improved procedure for estimating the metal surface area of supported copper catalysts. J. Catal. 1989, 118, 507–510. [Google Scholar] [CrossRef]














| HPW·6H2O Relative XRD Intensity (a.u.) | HPW·6H2O dp (nm) | Acid/Neutral Water Ratio (1700/1620 cm−1) | HPW/TiO2 Relative Raman Intensity (990/650 cm−1) | |
|---|---|---|---|---|
| 1.4HPW/Ti | 1.0 | 20.5 | 0.423 | 0.371 |
| 1.7HPW/Ti | 1.47 | 17.6 | 0.403 | 0.365 |
| 2.7HPW/Ti | 4.6 | 30.6 | 0.784 | 0.536 |
| Area | Area | H+/KU | |
|---|---|---|---|
| (LT-MT) | (HT) | ||
| 1.4HPW/Ti | 93.25 | 108.12 | 1.26 |
| 1.7HPW/Ti | 177.31 | 132.65 | 1.55 |
| 2.7HPW/Ti | 108.54 | 202.72 | 2.37 |
| HPW | - | 215.15 | 3.0 |
| Calcined | Reduced | |||||
|---|---|---|---|---|---|---|
| CuO | ZnO | HPA·3H2O | Cu | ZnO | Cu Surface Area | |
| dp (nm) | dp (nm) | dp (nm) | dp (nm) | dp (nm) | (m2/gcat) | |
| CZA-1.4HPW/Ti | 4.5 | 5.1 | 11.4 | 5.1 | 6.3 | 28.6 |
| CZA-1.7HPW/Ti | 4.4 | 5.3 | 4.4 | 5.4 | 6.1 | 35.5 |
| CZA-2.7HPW/Ti | 4.8 | 5.3 | 4.8 | 5.7 | 6.5 | 39.1 |
| CZA | 4.5 | 4.9 | - | 5.1 | 6.7 | 43.4 |
| CO Conversion (%) | Selectivity (%) | DME Time Yield | Deactivation Rate DME | |||
|---|---|---|---|---|---|---|
| Methanol | DME | CO2 | (μmol/min gcat) | (μmol/min gcat) | ||
| CZA | 17.6 | 98.9 | - | 1.1 | 967.3 * | 0.22 * |
| HZSM-5-CZA | 11.4 | 6.2 | 55.7 | 38.1 | 229.4 | 0.24 |
| CZA-1.4HPW/Ti | 14.5 | 97.4 | 2.5 | 0.1 | 12.3 | 0.06 |
| CZA-1.7HPW/Ti | 10.0 | 12.6 | 51.3 | 36.1 | 201.9 | 0.20 |
| CZA-2.7HPW/Ti | 11.0 | 6.4 | 53.0 | 40.6 | 243.0 | 0.14 |
| Cu | ZnO | |
|---|---|---|
| dp (nm) | dp (nm) | |
| CZA-1.4HPW/Ti | 5.1 | 6.3 |
| CZA-1.7HPW/Ti | 5.4 | 6.1 |
| CZA-2.7HPW/Ti | 5.7 | 6.5 |
| CZA | 5.1 | 6.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millán, E.; Mota, N.; Guil-López, R.; Pawelec, B.; García Fierro, J.L.; Navarro, R.M. Direct Synthesis of Dimethyl Ether from Syngas on Bifunctional Hybrid Catalysts Based on Supported H3PW12O40 and Cu-ZnO(Al): Effect of Heteropolyacid Loading on Hybrid Structure and Catalytic Activity. Catalysts 2020, 10, 1071. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091071
Millán E, Mota N, Guil-López R, Pawelec B, García Fierro JL, Navarro RM. Direct Synthesis of Dimethyl Ether from Syngas on Bifunctional Hybrid Catalysts Based on Supported H3PW12O40 and Cu-ZnO(Al): Effect of Heteropolyacid Loading on Hybrid Structure and Catalytic Activity. Catalysts. 2020; 10(9):1071. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091071
Chicago/Turabian StyleMillán, Elena, Noelia Mota, Rut Guil-López, Bárbara Pawelec, José L. García Fierro, and Rufino M. Navarro. 2020. "Direct Synthesis of Dimethyl Ether from Syngas on Bifunctional Hybrid Catalysts Based on Supported H3PW12O40 and Cu-ZnO(Al): Effect of Heteropolyacid Loading on Hybrid Structure and Catalytic Activity" Catalysts 10, no. 9: 1071. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091071