Structural Disorder of Mechanically Activated δ-MgCl2 Studied by Synchrotron X-ray Total Scattering and Vibrational Spectroscopy
Abstract
:1. Introduction
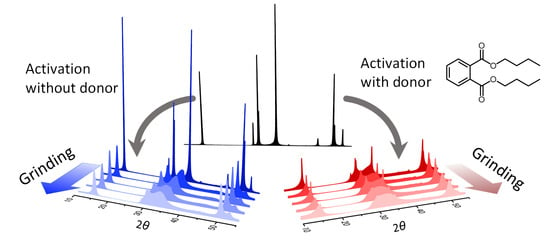
2. Results and Discussion
3. Materials and Methods
3.1. Sample Preparation
3.2. X-ray Total Scattering Measurement
3.3. PXRD Fitting
3.4. Acquisition and Fitting of PDF
3.5 Acquisition of FT-IR Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Busico, V. Giulio Natta and the development of stereoselective propene polymerization. In Polyolefins: 50 Years after Ziegler and Natta I Polyolefins: 50 Years after Ziegler and Natta I Polyethylene and Polypropylene; Advances in Polymer Science 257; Kaminsky, W., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2013; pp. 37–57. [Google Scholar]
- Bassi, I.W.; Polato, F.; Calcaterra, M.; Bart, J.C.J. A new layer structure of MgCl2 with hexagonal close packing of the chlorine atoms. Z. Krist. New Cryst. Struct. 1982, 159, 297–302. [Google Scholar] [CrossRef]
- Di Noto, V.; Bresadola, S. New synthesis of a highly active δ-MgCl2 for MgCl2/TiCl4/AlEt3 catalytic systems. Macromol. Chem. Phys. 1996, 197, 3827–3835. [Google Scholar] [CrossRef]
- Wada, T.; Takasao, G.; Piovano, A.; D’Amore, M.; Thakur, A.; Chammingkwan, P.; Bruzzese, P.C.; Terano, M.; Civalleri, B.; Bordiga, S.; et al. Revisiting the identity of δ-MgCl2: Part I. Structural disorder studied by synchrotron X-ray total scattering. J. Catal. 2020, 385, 76–86. [Google Scholar] [CrossRef]
- Zannetti, R.; Marega, C.; Marigo, A.; Martorana, A. Layer-lattices in Ziegler-Natta catalysts. J. Polym. Sci. Part. B Polym. Phys. 1988, 26, 2399–2412. [Google Scholar] [CrossRef]
- Busico, V.; Causà, M.; Cipullo, R.; Credendino, R.; Cutillo, F.; Friederichs, N.; Lamanna, R.; Segre, A.; Van Axel Castelli, V. Periodic DFT and high-resolution magic-angle-spinning (HR-MAS) 1H NMR investigation of the active surfaces of MgCl2-supported Ziegler-Natta catalysts. The MgCl2 matrix. J. Phys. Chem. C 2008, 112, 1081–1089. [Google Scholar] [CrossRef]
- Marigo, A.; Marega, C.; Zannetti, R.; Morini, G.; Ferrara, G. Small- and wide-angle X-ray scattering analysis of Ziegler-Natta catalysts: Structural disorder, surface area and activity. Eur. Polym. J. 2000, 36, 1921–1926. [Google Scholar] [CrossRef]
- Takasao, G.; Wada, T.; Thakur, A.; Chammingkwan, P.; Terano, M.; Taniike, T. Machine learning-aided structure determination for TiCl4–capped MgCl2 nanoplate of heterogeneous Ziegler-Natta catalyst. ACS Catal. 2019, 9, 2599–2609. [Google Scholar] [CrossRef]
- Blaakmeer, E.S.M.; Antinucci, G.; Busico, V.; van Eck, E.R.H.; Kentgens, A.P.M. Solid-state NMR investigations of MgCl2 catalyst support. J. Phys. Chem. C 2016, 120, 6063–6074. [Google Scholar] [CrossRef]
- Blaakmeer, E.S.M.; Antinucci, G.; Correa, A.; Busico, V.; van Eck, E.R.H.; Kentgens, A.P.M. Structural characterization of electron donors in Ziegler-Natta catalysts. J. Phys. Chem. C 2018, 122, 5525–5536. [Google Scholar] [CrossRef] [Green Version]
- Zorve, P.; Linnolahti, M. Saturation of magnesium dichloride crystallites by titanium tetrachloride. Surf. Sci. 2020, 699, 121627. [Google Scholar] [CrossRef]
- Breuza, E.; Antinucci, G.; Budzelaar, P.H.M.; Busico, V.; Correa, A.; Ehm, C. MgCl2-supported Ziegler-Natta catalysts: A DFT-D “flexible-cluster” approach to internal donor adducts. J. Phys. Chem. C 2018, 122, 9046–9053. [Google Scholar] [CrossRef]
- Credendino, R.; Pater, J.T.M.; Correa, A.; Morini, G.; Cavallo, L. Thermodynamics of formation of uncovered and dimethyl ether-covered MgCl2 crystallites. consequences in the structure of Ziegler-Natta heterogeneous catalysts. J. Phys. Chem. C 2011, 115, 13322–13328. [Google Scholar] [CrossRef]
- Suarez, S.; Di Noto, V.; Stallworth, P.E.; Abbrent, S.; Vittadello, M.; Alamgir, F.M.; Greenbaum, S.G.; Drain, C.M. Polymeric δ-MgCl2 nanoribbons. Inorg. Chim. Acta 2006, 359, 2513–2518. [Google Scholar]
- Piovano, A.; D’Amore, M.; Wada, T.; Bruzzese, P.C.; Takasao, G.; Thakur, A.; Chammingkwan, P.; Terano, M.; Civalleri, B.; Bordiga, S.; et al. Revisiting the identity of δ-MgCl2: Part II. Morphology and exposed surfaces studied by vibrational spectroscopies and DFT calculation. J. Catal. 2020, 387, 1–11. [Google Scholar] [CrossRef]
- Billinge, S.J.L. Nanoscale structural order from the atomic pair distribution function (PDF): There’s plenty of room in the middle. J. Solid State Chem. 2008, 181, 1695–1700. [Google Scholar] [CrossRef]
- Egami, T.; Billinge, S.J.L. (Eds.) Underneath the Bragg Peaks, 2nd ed.; Elsevier: Oxford, UK, 2012. [Google Scholar]
- Bae, S.; Jee, H.; Suh, H.; Kanematsu, M.; Shiro, A.; Machida, A.; Watanuki, T.; Shobu, T.; Morooka, S.; Geng, G.; et al. Analysis of atomistic structural deformation characteristics of calcium silicate hydrate in 53-year-old tricalcium silicate paste using atomic pair distribution function. Constr. Build. Mater. 2020, 237, 117714. [Google Scholar] [CrossRef]
- Christiansen, T.L.; Cooper, S.R.; Jensen, K.M.Ø. There’s no place like real-space: Elucidating size-dependent atomic structure of nanomaterials using pair distribution function analysis. Nanoscale Adv. 2020, 2, 2234–2254. [Google Scholar] [CrossRef]
- Taniike, T.; Funako, T.; Terano, M. Multilateral characterization for industrial Ziegler-Natta catalysts toward elucidation of structure–performance relationship. J. Catal. 2014, 311, 33–40. [Google Scholar] [CrossRef]
- Taniike, T.; Terano, M. The use of donors to increase the isotacticity of polypropylene. In Polyolefins: 50 Years after Ziegler and Natta I Polyolefins: 50 Years after Ziegler and Natta I Polyethylene and Polypropylene; Advances in Polymer Science 257; Kaminsky, W., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2013; pp. 81–97. [Google Scholar]
- Dutta, H.; Sahu, P.; Pradhan, S.K.; De, M. Microstructure characterization of polymorphic transformed ball-milled anatase TiO2 by Rietveld method. Mater. Chem. Phys. 2003, 77, 153–164. [Google Scholar] [CrossRef]
- Cui, B.Z.; Gabay, A.M.; Li, W.F.; Marinescu, M.; Liu, J.F.; Hadjipanayis, G.C. Anisotropic SmCo5 nanoflakes by surfactant-assisted high energy ball milling. J. Appl. Phys. 2010, 107, 105–108. [Google Scholar] [CrossRef]
- Shin, H.; Lee, S.; Suk Jung, H.; Kim, J.B. Effect of ball size and powder loading on the milling efficiency of a laboratory-scale wet ball mill. Ceram. Int. 2013, 39, 8963–8968. [Google Scholar] [CrossRef]
- Chammingkwan, P.; Thang, V.Q.; Terano, M.; Taniike, T. MgO/MgCl2/TiCl4 Core–shell catalyst for establishing structure–performance relationship in Ziegler-Natta olefin polymerization. Top. Catal. 2014, 57, 911–917. [Google Scholar] [CrossRef]
- Potapov, A.G.; Bukatov, G.D.; Zakharov, V.A. Drift study of internal donors in supported Ziegler-Natta catalysts. J. Mol. Catal. A Chem. 2006, 246, 248–254. [Google Scholar] [CrossRef]
- Piovano, A.; D’Amore, M.; Thushara, K.S.; Groppo, E. Spectroscopic evidences for TiCl4/Donor complexes on the surface of MgCl2-supported Ziegler-Natta catalysts. J. Phys. Chem. C 2018, 122, 5615–5626. [Google Scholar] [CrossRef]
- Cheruvathur, A.V.; Langner, E.H.G.; Niemantsverdriet, J.W.H.; Thüne, P.C. In situ ATR-FTIR studies on MgCl2-diisobutyl phthalate interactions in thin film Ziegler-Natta catalysts. Langmuir 2012, 28, 2643–2651. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, S.; Makwana, U.; Patankar, R.B.; Gupta, V.K. Influence of internal donors on the performance and structure of MgCl2 supported titanium catalysts for propylene polymerization. Macromol. Chem. Phys. 2009, 210, 69–76. [Google Scholar] [CrossRef]
- Kuklin, M.S.; Bazhenov, A.S.; Denifl, P.; Leinonen, T.; Linnolahti, M.; Pakkanen, T.A. Stabilization of magnesium dichloride surface defects by mono- and bidentate donors. Surf. Sci. 2015, 635. [Google Scholar] [CrossRef]
- Vanka, K.; Singh, G.; Iyer, D.; Gupta, V.K. DFT study of Lewis base interactions with the MgCl2 surface in the Ziegler-Natta catalytic system: Expanding the role of the donors. J. Phys. Chem. C 2010, 114, 15771–15781. [Google Scholar] [CrossRef]
- Shetty, S. Synergistic, reconstruction and bonding effects during the adsorption of internal electron donors and TiCl4 on MgCl2 surface: A periodic-DFT investigation. Surf. Sci. 2016, 653, 55–65. [Google Scholar] [CrossRef]
- Proffen, T.; Neder, R.B. DISCUS, a program for diffuse scattering and defect structure simulations—Update. J. Appl. Cryst. 1999, 32, 838–839. [Google Scholar] [CrossRef]
- Partin, D.E.; O’Keeffe, M. The structures and crystal chemistry of magnesium chloride and cadmium chloride. J. Solid State Chem. 1991, 95, 176–183. [Google Scholar] [CrossRef]
- Scardi, P.; Billinge, S.J.L.; Neder, R.; Cervellino, A. Celebrating 100 years of the Debye scattering equation. Acta Cryst. Sect. A Found. Adv. 2016, 72, 589–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, X.; Thompson, J.W.; Billinge, S.J.L. PDFgetX2: A GUI-driven program to obtain the pair distribution function from X-ray powder diffraction data. J. Appl. Cryst. 2004, 37, 678. [Google Scholar] [CrossRef] [Green Version]
- Božin, E.S.; Billinge, S.J.L.; Proffen, T.; Farrow, C.L.; Juhas, P.; Bryndin, D.; Liu, J.W.; Bloch, J. PDFfit2 and PDFgui: Computer programs for studying nanostructure in crystals. J. Phys. Condens. Matter 2007, 19, 335219. [Google Scholar]




| Sample Name | Pc [%] | La × Lb [nm] | Lc [nm] |
|---|---|---|---|
| Pristine MgCl2 | 100 1 | 61.5 2 | 72.4 2 |
| Dry-3 | 91 3 | 56.8 1 | 54.2 1 |
| Dry-6 | 58 | 12.7 × 12.7 | 13.3 |
| Dry-12 | 55 | 12.7 × 12.7 | 13.3 |
| Dry-24 | 40 | 10.9 × 10.9 | 11.5 |
| Dry-48 | 40 | 10.9 × 10.9 | 11.5 |
| EB-3 | 88 3 | 38.7 2 | 41.5 2 |
| EB-6 | 58 | 14.5 × 14.5 | 13.3 |
| EB-12 | 58 | 14.5 × 14.5 | 13.3 |
| EB-24 | 55 | 10.9 × 10.9 | 10.3 |
| EB-48 | 46 | 9.1 × 9.1 | 8.0 |
| DBP-3 | 55 | 12.7 × 12.7 | 13.3 |
| DBP-6 | 34 | 9.1 × 9.1 | 9.7 |
| DBP-12 | 37 | 10.9 × 10.9 | 8.0 |
| DBP-24 | 31 | 9.1 × 3.6 | 6.2 |
| DBP-48 | 31 | 9.1 × 3.6 | 6.2 |
| Tol-6 | 82 | 29.1 × 29.1 | 29.2 |
| Tol-12 | 64 | 16.4 × 16.4 | 16.2 |
| Tol-24 | 46 | 12.7 × 12.7 | 11.5 |
| Tol-48 | 31 | 9.1 × 9.1 | 8.0 |
| Mg(OEt)2-based catalyst | 25 | 7.6 × 3.3 | 0.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, T.; Thakur, A.; Chammingkwan, P.; Terano, M.; Taniike, T.; Piovano, A.; Groppo, E. Structural Disorder of Mechanically Activated δ-MgCl2 Studied by Synchrotron X-ray Total Scattering and Vibrational Spectroscopy. Catalysts 2020, 10, 1089. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091089
Wada T, Thakur A, Chammingkwan P, Terano M, Taniike T, Piovano A, Groppo E. Structural Disorder of Mechanically Activated δ-MgCl2 Studied by Synchrotron X-ray Total Scattering and Vibrational Spectroscopy. Catalysts. 2020; 10(9):1089. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091089
Chicago/Turabian StyleWada, Toru, Ashutosh Thakur, Patchanee Chammingkwan, Minoru Terano, Toshiaki Taniike, Alessandro Piovano, and Elena Groppo. 2020. "Structural Disorder of Mechanically Activated δ-MgCl2 Studied by Synchrotron X-ray Total Scattering and Vibrational Spectroscopy" Catalysts 10, no. 9: 1089. https://0-doi-org.brum.beds.ac.uk/10.3390/catal10091089