Highly Porous SnO2/TiO2 Heterojunction Thin-Film Photocatalyst Using Gas-Flow Thermal Evaporation and Atomic Layer Deposition
Abstract
:1. Introduction
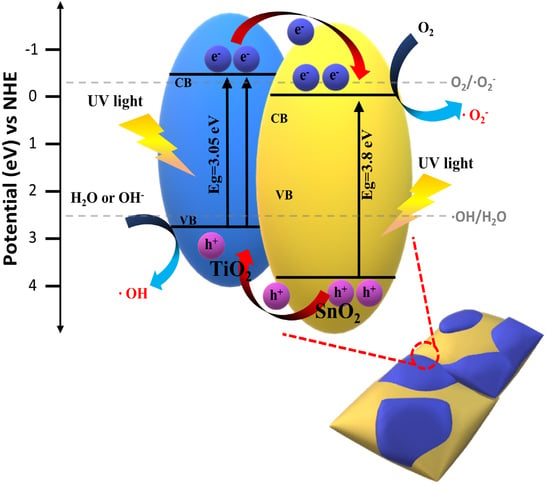
2. Results and Discussion
3. Experimental Section
3.1. Chemical and Substrate
3.2. Preparation of Porous SnO2 Nanoform
3.3. Preparation of the Porous SnO2/TiO2 Heterostructure
3.4. Evaluation of Photocatalytic Activity
3.5. Materials Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rasheed, T.; Bilal, M.; Nabeel, F.; Adeel, M.; Iqbal, H.M.N. Environmentally-related contaminants of high concern: Potential sources and analytical modalities for detection, quantification, and treatment. Environ. Int. 2019, 122, 52–66. [Google Scholar] [CrossRef]
- Khan, S.; Malik, A. Toxicity evaluation of textile effluents and role of native soil bacterium in biodegradation of a textile dye. Sci. Pollut. Res. 2018, 25, 4446–4458. [Google Scholar] [CrossRef] [PubMed]
- Hodeges, B.C.; Cates, E.L.; Kim, J.-H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Mashuri, S.I.S.; Ibrahim, M.L.; Kasim, M.F.; Mastuli, M.S.; Rashid, U.; Abrullah, A.H.; Islam, A.; Mijan, N.A.; Tan, Y.H.; Mansir, N.; et al. Photocatalysis for organic wastewater treatment: From the basis to current challenges for society. Catalysts 2020, 10, 1260. [Google Scholar] [CrossRef]
- Chan, S.H.S.; Wu, T.Y.; Juna, J.C.; Teh, C.Y. Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. J. Chem. Technol. Biotechnol. 2011, 86, 1130–1158. [Google Scholar] [CrossRef]
- Martini, J.; Orge, C.A.; Faria, J.L.; Rereira, M.F.R.; Soares, O.S.G.P. Catalytic advanced oxidation processes for sulfamethoxazole degradation. Appl. Sci. 2019, 9, 2652. [Google Scholar] [CrossRef] [Green Version]
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Puma, G.L.; Quan, X.; et al. The Technology horizon for photocatalytic water treatment: Sunrise or sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhan, W.; Wang, F.; Safdar, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Understanding hydroxyl radical (·OH) generation processes in photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef] [Green Version]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy. Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, Q.; Wu, A.; Jiang, M.; Liang, Z.; Jiang, B.; Fu, H. Cost-effective large-scale synthesis of ZnO photocatalyst with excellent performance for dye photodegradation. Chem. Commu. 2012, 48, 2858–2860. [Google Scholar] [CrossRef]
- Vaseem, M.; Umar, A.; Hahn, Y.B.; Kim, D.H.; Lee, K.S.; Jang, J.S.; Lee, J.S. Flower-shaped CuO nanostructures: Structural, photocatalytic and XANES studies. Catal. Commun. 2008, 10, 11–16. [Google Scholar] [CrossRef]
- Ramesh, M.; Rao, M.P.C.; Anandan, S.; Nagaraja, H. Adsorption and photocatalytic properties of NiO nanoparticles synthesized via a thermal decomposition process. J. Mater. Res. 2018, 33, 601–610. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, M.; Li, D.; Fang, F.; Huang, W. Engineering self-doped surface defects of anatase TiO2 nanosheets for enhanced photocatalytic efficiency. Appl. Surf. Sci. 2021, 540, 148330. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, M.; Song, D.; Pan, X.; Fu, J.; Zhou, J.; Ma, S.; Wang, T. Highly porous SnO2/TiO2 electrospun nanofibers with high photocatalytic activities. Ceram. Int. 2014, 40, 10383–10393. [Google Scholar] [CrossRef]
- Cheng, H.-E.; Lin, C.-Y.; Hsu, C.-M. Fabrication of SnO2-TiO2 core-shell nanopillar array films for enhanced photocatalytic activity. Appl. Sur. Sci. 2017, 396, 393–399. [Google Scholar] [CrossRef]
- Malagutti, A.R.; Mourão, H.A.J.L.; Garbin, J.R.; Ribeiro, C. Deposition of TiO2 and Ag:TiO2 thin films by the polymeric precursor method and their application in the photodegradation of textile dyes. Appl. Catal. B 2009, 90, 205–212. [Google Scholar] [CrossRef]
- Dey, N.K.; Kim, M.J.; Kim, K.-D.; Seo, H.O.; Kim, D.H.; Kim, Y.D.; Lim, D.C.; Lee, K.H. Adsorption and photocatalytic degradation of methylene blue over TiO2 films on carbon fiber prepared by atomic layer deposition. J. Mol. Catal. A Chem. 2011, 337, 33–38. [Google Scholar] [CrossRef]
- Arekhi, M.; Jamshidi, M. Influences of inorganic binder on photocatalytic oxidation (PCO) and degradation of nano/micro TiO2 containing acrylic composites. Prog. Org. Coat. 2018, 115, 1–8. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Y.; Wei, Z.; Yang, S.; He, H.; Sun, C. Fabrication of a novel p-n heterojunction photocatalyst n-BiVo4@p-MoS2 with core-shell structure and its excellent visible-light photocatalytic reduction and oxidation activities. Appl. Catal. B 2016, 185, 242–252. [Google Scholar] [CrossRef]
- Wang, H.-B.; Ma, F.; Sun, Y.-S.; Zhou, L.; Zeng, D.-J.; Qin, Y.; Xu, Y.-K.; Chen, Y.-N.; Xu, K.-W.; Ma, D.-Y. Band bending and valence band shifting of sub-monolayer TiO2 functionalized SnO2 nanowires. J. Mater. Sci. Mater. Electron. 2020, 31, 637–643. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, K.; RZhuang, R.; Wu, K.; Liao, Q.; Zhong, S.; Yue, H.; Liu, C.; Tang, S.; Liang, B. Hydroxyl-mediated formation of highly dispersed SnO2/TiO2 heterojunction via pulsed chemical vapor deposition to enhance photocatalytic activity. Ind. Eng. Chem. Res. 2019, 58, 14655–14663. [Google Scholar] [CrossRef]
- Du, H.; Yao, P.; Sun, Y.; Wang, J.; Wang, H.; Yu, N. Electrospinning hetero-nanofibers In2O3/SnO2 of homotype heterojunction with high gas sensing activity. Sensors 2017, 17, 1822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ma, Y.; Bu, X.; Wu, Q.; Hang, Z.; Dong, Z.; Wu, X. Facile one-step synthesis of TiO2/Ag/SnO2 ternary heterostructures with enhanced visible light photocatalytic activity. Sci. Rep. 2018, 8, 10532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghobadi, A.; Ulusoy, T.G.; Garifullin, R.; Guler, M.O.; Okyay, A.K. A heterojunction design of single layer hole tunneling ZnO passivation wrapping around TiO2 nanowires for superior photocatalytic performance. Sci. Rep. 2016, 6, 30587. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Li, W.; Yang, Q.; Hou, Q.; Wei, L.; Liu, L.; Huang, F.; Ju, M. Enhancement of photocatalytic performance with the use of noble-metal-decorated TiO2 nanocrystals as highly active catalysts for aerobic oxidation under visible. Appl. Catal. B 2017, 210, 352–367. [Google Scholar] [CrossRef]
- Wang, X.; Ni, Q.; Zeng, D.; Liao, G.; Wen, Y.; Shan, B.; Xie, C. BiOCl/TiO2 heterojunction network with high energy facet exposed for highly efficient photocatalytic degradation of benzene. Appl. Surf. Sci. 2017, 296, 590–598. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Li, Y.; Yang, M.; Chang, Y.-Q.; Zhang, J.-P.; Sun, Z.; Wang, Y. Enhanced light absorption in porous particles for Ultra-NIR-Sensitive biomaterials. ACS Macro. Lett. 2015, 4, 392–397. [Google Scholar] [CrossRef]
- Kim, S.P.; Choi, M.Y.; Choi, H.C. Photocatalytic activity of SnO2 nanoaprticles in methylene blue degradation. Mater. Res. Bull. 2016, 74, 85–89. [Google Scholar] [CrossRef]
- Lv, L.; Bai, X.; Ye, Z. Construction of N-doped TiO2/SnO2 heterostructured microspheres with dominant {001} facets for enhanced photocatalytic properties. CrystEngComm 2016, 39, 7580–7589. [Google Scholar] [CrossRef]
- Alagarasi, A.; Rajalakshmi, P.U.; Shanthi, K.; Selvam, P. Solar-light driven photocatalytic activity of mesoporous nanocrystalline TiO2, SnO2, and TiO2-SnO2 composites. Mater. Today Sustain. 2019, 5, 100016. [Google Scholar] [CrossRef]
- Park, Y.M.; Hwang, S.H.; Lim, H.; Lee, H.-N.; Kim, H.-J. Scalable and Versatile Fabrication of Metallic Nanofoam Films with Controllable Nanostructure Using Ar-Assisted Thermal Evaporation. Chem. Mater. 2020, 33, 205–211. [Google Scholar] [CrossRef]
- Chang, H.-K.; Ko, D.-S.; Cho, D.-H.; Kim, S.; Lee, H.-N.; Lee, H.S.; Kim, H.-J.; Park, T.J.; Park, Y.M. Enhanced response of the photoactive gas sensor on formaldehyde using porous SnO2@TiO2 heterostructure driven by gas-flow thermal evaporation and atomic layer deposition. Ceram. Int. 2021, 47, 5958–5992. [Google Scholar] [CrossRef]








| MB | SnO2 | SnO2/TiO2-10cyc | SnO2/TiO2-25cyc | SnO2/TiO2-50cyc | SnO2/TiO2-100cyc | |
|---|---|---|---|---|---|---|
| Photo-catalytic efficiency (%) | <1 | 60 | 73 | 91 | 99 | 81 |
| K (min−1) | N/A | 0.0030 | 0.0042 | 0.0078 | 0.013 | 0.0056 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Chang, H.-K.; Kim, K.B.; Kim, H.-J.; Lee, H.-N.; Park, T.J.; Park, Y.M. Highly Porous SnO2/TiO2 Heterojunction Thin-Film Photocatalyst Using Gas-Flow Thermal Evaporation and Atomic Layer Deposition. Catalysts 2021, 11, 1144. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11101144
Kim S, Chang H-K, Kim KB, Kim H-J, Lee H-N, Park TJ, Park YM. Highly Porous SnO2/TiO2 Heterojunction Thin-Film Photocatalyst Using Gas-Flow Thermal Evaporation and Atomic Layer Deposition. Catalysts. 2021; 11(10):1144. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11101144
Chicago/Turabian StyleKim, Sungjin, Hyeon-Kyung Chang, Kwang Bok Kim, Hyun-Jong Kim, Ho-Nyun Lee, Tae Joo Park, and Young Min Park. 2021. "Highly Porous SnO2/TiO2 Heterojunction Thin-Film Photocatalyst Using Gas-Flow Thermal Evaporation and Atomic Layer Deposition" Catalysts 11, no. 10: 1144. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11101144