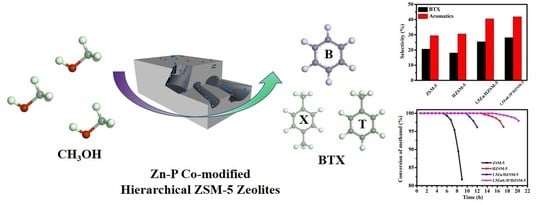
Zn-P Co-Modified Hierarchical ZSM-5 Zeolites Directly Synthesized via Dry Gel Conversion for Enhanced Methanol to Aromatics Reaction
Abstract
:1. Introduction
2. Results and Discussions
2.1. Sample Characterizations
2.2. Catalytic Performance in Methanol to Aromatics (MTA) Reaction
3. Materials and Methods
3.1. Catalyst Preparation
3.1.1. The Preparation of ZSM-5 Zeolites via Dry Gel Conversion (DGC)
3.1.2. The Preparation of Hierarchical ZSM-5 Zeolites via the Destructive Method
3.1.3. The Preparation of Zn-Modified or Zn-P Co-Modified Hierarchical ZSM-5 Zeolites via Impregnation Method
3.2. Characterization
3.3. MTA Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gao, P.; Xu, J.; Qi, G.; Wang, C.; Wang, Q.; Zhao, Y.; Zhang, Y.; Feng, N.; Zhao, X.; Li, J. A mechanistic study of methanol-to-aromatics reaction over Ga-modified ZSM-5 zeolites: Understanding the dehydrogenation process. ACS Catal. 2018, 8, 9809–9820. [Google Scholar] [CrossRef]
- Olah, G.A. Beyond oil and gas: The methanol economy. Angew. Chem. Int. Ed. 2005, 44, 2636–2639. [Google Scholar] [CrossRef]
- Niu, X.; Gao, J.; Miao, Q.; Dong, M.; Wang, G.; Fan, W.; Qin, Z.; Wang, J. Influence of preparation method on the performance of Zn-containing HZSM-5 catalysts in methanol-to-aromatics. Microporous Mesoporous Mater. 2014, 197, 252–261. [Google Scholar] [CrossRef]
- Kim, S.D.; Noh, S.H.; Seong, K.H.; Kim, W.J. Compositional and kinetic study on the rapid crystallization of ZSM-5 in the absence of organic template under stirring. Microporous Mesoporous Mater. 2004, 72, 185–192. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.B.; Yang, N.; Jiao, W.Q.; Wang, Y.M.; He, M.-Y. Dry-gel synthesis of shaped binderless zeolites composed of nanosized ZSM-5. Solid State Sci. 2013, 20, 1–7. [Google Scholar] [CrossRef]
- Wu, G.; Hei, F.; Guan, N.; Li, L. Oxidative dehydrogenation of propane with nitrous oxide over Fe–MFI prepared by ion-exchange: Effect of acid post-treatments. Catal. Sci. Technol. 2013, 3, 1333–1342. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Qi, G.; Guo, Q.; Pan, S.; Meng, X.; Xu, J.; Deng, F.; Fan, F.; Feng, Z. Sustainable synthesis of zeolites without addition of both organotemplates and solvents. J. Am. Chem. Soc. 2014, 136, 4019–4025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, Q.; Lei, C.; Pan, S.; Bian, C.; Wang, L.; Meng, X.; Xiao, F.-S. Solvent-free and mesoporogen-free synthesis of mesoporous aluminosilicate ZSM-5 zeolites with superior catalytic properties in the methanol-to-olefins reaction. Ind. Eng. Chem. Res. 2017, 56, 1450–1460. [Google Scholar] [CrossRef]
- Yarulina, I.; Chowdhury, A.D.; Meirer, F.; Weckhuysen, B.M.; Gascon, J. Recent trends and fundamental insights in the methanol-to-hydrocarbons process. Nat. Catal. 2018, 1, 398–411. [Google Scholar] [CrossRef]
- Wang, N.; Qian, W.; Shen, K.; Su, C.; Wei, F. Bayberry-like ZnO/MFI zeolite as high performance methanol-to-aromatics catalyst. Chem. Commun. 2016, 52, 2011–2014. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.; Wells, R.P.; Hutchings, G.J. Conversion of methanol to hydrocarbons over Ga2O3/H-ZSM-5 and Ga2O3/WO3 catalysts. J. Catal. 2002, 205, 358–365. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, J.; Zhang, K.; Feng, W.; Liu, S.; Ding, C.; Liu, P. Promoted effect of zinc–nickel bimetallic oxides supported on HZSM-5 catalysts in aromatization of methanol. J. Energy Chem. 2017, 26, 540–548. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Zan, W.; Bai, X.; Wang, G.; Wu, W. Synthesis of microscale and nanoscale ZSM-5 zeolites: Effect of particle size and acidity of Zn modified ZSM-5 zeolites on aromatization performance. Catal. Sci. Technol. 2017, 7, 1943–1952. [Google Scholar] [CrossRef]
- Conte, M.; Lopez-Sanchez, J.A.; He, Q.; Morgan, D.J.; Ryabenkova, Y.; Bartley, J.K.; Carley, A.F.; Taylor, S.H.; Kiely, C.J.; Khalid, K. Modified zeolite ZSM-5 for the methanol to aromatics reaction. Catal. Sci. Technol. 2012, 2, 105–112. [Google Scholar] [CrossRef]
- Gong, Q.; Fang, T.; Xie, Y.; Zhang, R.; Liu, M.; Barzagli, F.; Li, J.; Hu, Z.; Zhu, Z. High-Efficiency Conversion of Methanol to BTX Aromatics Over a Zn-Modified Nanosheet-HZSM-5 Zeolite. Ind. Eng. Chem. Res. 2021, 60, 1633–1641. [Google Scholar] [CrossRef]
- Ono, Y.; Adachi, H.; Senoda, Y. Selective conversion of methanol into aromatic hydrocarbons over zinc-exchanged ZSM-5 zeolites. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1988, 84, 1091–1099. [Google Scholar] [CrossRef]
- Kolyagin, Y.G.; Ordomsky, V.; Khimyak, Y.; Rebrov, A.; Fajula, F.; Ivanova, I. Initial stages of propane activation over Zn/MFI catalyst studied by in situ NMR and IR spectroscopic techniques. J. Catal. 2006, 238, 122–133. [Google Scholar] [CrossRef]
- Pan, T.; Wu, Z.; Zhou, K. In Situ Incorporation of Zn into Hierarchical ZSM-5 Zeolites for Olefin Hydroisomerization. Ind. Eng. Chem. Res. 2020, 59, 12371–12380. [Google Scholar] [CrossRef]
- Zhang, J.; Qian, W.; Kong, C.; Wei, F. Increasing para-xylene selectivity in making aromatics from methanol with a surface-modified Zn/P/ZSM-5 catalyst. ACS Catal. 2015, 5, 2982–2988. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, X.; Zhang, S.; Cheng, M.; Yu, M.; Wang, G.; Li, C. Selective production of para-xylene and light olefins from methanol over the mesostructured Zn–Mg–P/ZSM-5 catalyst. Catal. Sci. Technol. 2019, 9, 316–326. [Google Scholar] [CrossRef]
- Miyake, K.; Hirota, Y.; Ono, K.; Uchida, Y.; Tanaka, S.; Nishiyama, N. Direct and selective conversion of methanol to para-xylene over Zn ion doped ZSM-5/silicalite-1 core-shell zeolite catalyst. J. Catal. 2016, 342, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Pan, D.; Song, X.; Yang, X.; Gao, L.; Wei, R.; Zhang, J.; Xiao, G. Efficient and selective conversion of methanol to para-xylene over stable H [Zn, Al] ZSM-5/SiO2 composite catalyst. Appl. Catal. A Gen. 2018, 557, 15–24. [Google Scholar] [CrossRef]
- Sotelo, J.L.; Uguina, M.A.; Valverde, J.L.; Serrano, D.P. Kinetics of toluene alkylation with methanol over magnesium-modified ZSM-5. Ind. Eng. Chem. Res. 1993, 32, 2548–2554. [Google Scholar] [CrossRef]
- Janardhan, H.; Shanbhag, G.; Halgeri, A. Shape-selective catalysis by phosphate modified ZSM-5: Generation of new acid sites with pore narrowing. Appl. Catal. A Gen. 2014, 471, 12–18. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ishida, A.; Okajima, M.; Niwa, M. Modification of HZSM-5 by CVD of various silicon compounds and generation of para-selectivity. J. Catal. 1996, 161, 387–392. [Google Scholar] [CrossRef]
- Xue, B.; Zhang, G.; Liu, N.; Xu, J.; Shen, Q.; Li, Y. Highly selective synthesis of para-diethylbenzene by alkylation of ethylbenzene with diethyl carbonate over boron oxide modified HZSM-5. J. Mol. Catal. A Chem. 2014, 395, 384–391. [Google Scholar] [CrossRef]
- Ni, Y.; Zhu, W.; Liu, Z. Formaldehyde intermediate participating in the conversion of methanol to aromatics over zinc modified H-ZSM-5. J. Energy Chem. 2021, 54, 174–178. [Google Scholar] [CrossRef]
- Teketel, S.; Skistad, W.; Benard, S.; Olsbye, U.; Lillerud, K.P.; Beato, P.; Svelle, S. Shape selectivity in the conversion of methanol to hydrocarbons: The catalytic performance of one-dimensional 10-ring zeolites: ZSM-22, ZSM-23, ZSM-48, and EU-1. ACS Catal. 2012, 2, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Peng, P.; Gao, X.-H.; Yan, Z.-F.; Mintova, S. Diffusion and catalyst efficiency in hierarchical zeolite catalysts. Natl. Sci. Rev. 2020, 7, 1726–1742. [Google Scholar] [CrossRef]
- Xue, T.; Chen, L.; Wang, Y.M.; He, M.-Y. Seed-induced synthesis of mesoporous ZSM-5 aggregates using tetrapropylammonium hydroxide as single template. Microporous Mesoporous Mater. 2012, 156, 97–105. [Google Scholar] [CrossRef]
- Chal, R.; Gérardin, C.; Bulut, M.; van Donk, S. Overview and industrial assessment of synthesis strategies towards zeolites with mesopores. ChemCatChem 2011, 3, 67–81. [Google Scholar] [CrossRef]
- Milina, M.; Mitchell, S.; Michels, N.-L.; Kenvin, J.; Pérez-Ramírez, J. Interdependence between porosity, acidity, and catalytic performance in hierarchical ZSM-5 zeolites prepared by post-synthetic modification. J. Catal. 2013, 308, 398–407. [Google Scholar] [CrossRef]
- Sun, H.; Peng, P.; Wang, Y.; Li, C.; Subhan, F.; Bai, P.; Xing, W.; Zhang, Z.; Liu, Z.; Yan, Z. Preparation, scale-up and application of meso-ZSM-5 zeolite by sequential desilication–dealumination. J. Porous Mater. 2017, 24, 1513–1525. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Chen, H.; Sun, C.; Meng, F.; Gao, F.; Wang, X. Direct synthesis of hierarchical ZnZSM-5 with addition of CTAB in a seeding method and improved catalytic performance in methanol to aromatics reaction. Catal. Today 2018, 316, 91–98. [Google Scholar] [CrossRef]
- Wang, L.; Sang, S.; Meng, S.; Zhang, Y.; Qi, Y.; Liu, Z. Direct synthesis of Zn-ZSM-5 with novel morphology. Mater. Lett. 2007, 61, 1675–1678. [Google Scholar] [CrossRef]
- Haase, M.; Weller, H.; Henglein, A. Photochemistry and radiation chemistry of colloidal semiconductors. 23. Electron storage on zinc oxide particles and size quantization. J. Phys. Chem. 1988, 92, 482–487. [Google Scholar] [CrossRef]
- Groen, J.C.; Moulijn, J.A.; Pérez-Ramírez, J. Decoupling mesoporosity formation and acidity modification in ZSM-5 zeolites by sequential desilication–dealumination. Microporous Mesoporous Mater. 2005, 87, 153–161. [Google Scholar] [CrossRef]
- Lee, S.; Choi, M. Unveiling coke formation mechanism in MFI zeolites during methanol-to-hydrocarbons conversion. J. Catal. 2019, 375, 183–192. [Google Scholar] [CrossRef]







| Relative Crystallinity | SBET a | Smicro b | Smeso c | Vtotal b | Vmicro b | Vmeso d | |
|---|---|---|---|---|---|---|---|
| (%) | (m2/g) | (m2/g) | (m2/g) | (cm3/g) | (cm3/g) | (cm3/g) | |
| ZSM-5 | 100% | 262 | 206 | 56 | 0.182 | 0.108 | 0.079 |
| HZSM-5 | 49.9 | 311 | 167 | 144 | 0.311 | 0.088 | 0.244 |
| 0.5Zn/HZSM-5 | 52.1 | 297 | 164 | 133 | 0.302 | 0.086 | 0.238 |
| 1.0Zn/HZSM-5 | 52.5 | 289 | 160 | 129 | 0.293 | 0.084 | 0.229 |
| 1.5Zn/HZSM-5 | 54.8 | 282 | 154 | 128 | 0.294 | 0.081 | 0.235 |
| 2.0Zn/HZSM-5 | 52.2 | 271 | 154 | 117 | 0.280 | 0.081 | 0.219 |
| 1.5Zn0.1P/HZSM-5 | 50.5 | 299 | 144 | 155 | 0.312 | 0.076 | 0.258 |
| 1.5Zn0.3P/HZSM-5 | 49.5 | 314 | 159 | 155 | 0.321 | 0.084 | 0.259 |
| 1.5Zn0.6P/HZSM-5 | 49.5 | 332 | 167 | 163 | 0.329 | 0.088 | 0.262 |
| 1.5Zn1.0P/HZSM-5 | 32.2 | 363 | 169 | 194 | 0.348 | 0.089 | 0.277 |
| Samples | Py-IR (μmol/g) | NH3-TPD (μmol/g) | |||||
|---|---|---|---|---|---|---|---|
| Brønsted a | Lewis a | L/B b | Total c | Strong d | Medium d | Weak d | |
| HZSM-5 | 262 | 114 | 0.44 | 761.6 | 212.7 | 174.3 | 374.7 |
| 0.5Zn/HZSM-5 | 246 | 204 | 0.83 | 807.1 | 180.9 | 223.8 | 402.4 |
| 1.0Zn/HZSM-5 | 164 | 222 | 1.35 | 799.3 | 173.5 | 240.1 | 385.7 |
| 1.5Zn/HZSM-5 | 161 | 252 | 1.57 | 816.5 | 177.3 | 245.2 | 394.0 |
| 2.0Zn/HZSM-5 | 159 | 278 | 1.75 | 818 | 152.6 | 257.9 | 407.5 |
| 1.5Zn0.1P/HZSM-5 | 153 | 303 | 1.98 | 834.3 | 173.3 | 254.3 | 406.7 |
| 1.5Zn0.3P/HZSM-5 | 146 | 319 | 2.19 | 966.2 | 244.9 | 266.2 | 455 |
| 1.5Zn0.6P/HZSM-5 | 141 | 300 | 2.13 | 846.2 | 180.4 | 223.4 | 442.4 |
| 1.5Zn1.0P/HZSM-5 | 134 | 284 | 2.12 | 782.7 | 175.1 | 208.9 | 398.7 |
| Sample | B (%) | T (%) | X (%) | BTX (%) | C9+ (%) | Aromatics (%) |
|---|---|---|---|---|---|---|
| ZSM-5 | 1.01 | 6.41 | 13.17 | 20.59 | 8.82 | 29.41 |
| HZSM-5 | 0.25 | 3.59 | 14.19 | 18.03 | 12.53 | 30.56 |
| 0.5Zn/HZSM-5 | 0.36 | 4.72 | 14.75 | 19.83 | 13.02 | 32.85 |
| 1.0Zn/HZSM-5 | 0.32 | 5.17 | 16.57 | 22.06 | 14.49 | 36.55 |
| 1.5Zn/HZSM-5 | 0.39 | 5.84 | 19.17 | 25.40 | 15.11 | 40.51 |
| 2.0Zn/HZSM-5 | 0.29 | 4.75 | 15.15 | 20.19 | 13.04 | 33.23 |
| 1.5Zn0.1P/HZSM-5 | 0.47 | 6.03 | 19.15 | 25.65 | 15.04 | 40.69 |
| 1.5Zn0.3P/HZSM-5 | 0.53 | 6.62 | 20.97 | 28.12 | 13.76 | 41.88 |
| 1.5Zn0.6P/HZSM-5 | 0.49 | 5.56 | 19.61 | 25.66 | 11.97 | 37.63 |
| 1.5Zn1.0P/HZSM-5 | 0.50 | 5.87 | 18.62 | 24.99 | 9.13 | 34.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, Z.; Dai, C.; Du, N.; Li, T.; Wang, R.; Peng, P.; Sun, H. Zn-P Co-Modified Hierarchical ZSM-5 Zeolites Directly Synthesized via Dry Gel Conversion for Enhanced Methanol to Aromatics Reaction. Catalysts 2021, 11, 1388. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111388
Wang Y, Li Z, Dai C, Du N, Li T, Wang R, Peng P, Sun H. Zn-P Co-Modified Hierarchical ZSM-5 Zeolites Directly Synthesized via Dry Gel Conversion for Enhanced Methanol to Aromatics Reaction. Catalysts. 2021; 11(11):1388. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111388
Chicago/Turabian StyleWang, Youhe, Zhihong Li, Chang Dai, Ningning Du, Tingting Li, Risheng Wang, Peng Peng, and Hongman Sun. 2021. "Zn-P Co-Modified Hierarchical ZSM-5 Zeolites Directly Synthesized via Dry Gel Conversion for Enhanced Methanol to Aromatics Reaction" Catalysts 11, no. 11: 1388. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11111388