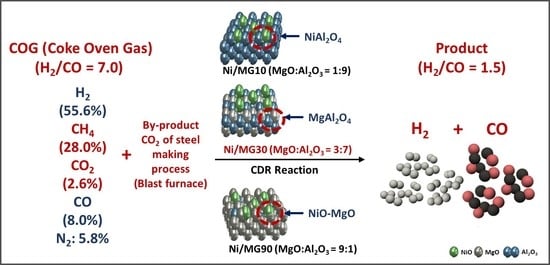
CO2 Reforming of CH4 Using Coke Oven Gas over Ni/MgO-Al2O3 Catalysts: Effect of the MgO:Al2O3 Ratio
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Reaction Results
3. Materials and Methods
3.1. Preparation of Catalysts
3.2. Catalyst Characterization
3.3. Catalyst activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Adoption of the Paris Agreement, L.9; UNFCCC: France, Paris, 2015; pp. 2–32.
- Rogelj, J.; den Elzen, M.; Höhne, N.; Fransen, T.; Fekete, H.; Winkler, H.; Schaeffer, R.; Sha, F.; Riahi, K.; Meinshausen, M. Paris agreement climate proposals need a boost to keep warming well below 2 degrees Celsius. Nature 2016, 534, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Energy Technology Perspectives 2016: Towards Sustainable Urban Energy Systems; IEA: France, Paris, 2016; pp. 25–76.
- Umar, M.; Ji, X.; Kirikkaleli, D.; Alola, A.A. The imperativeness of environmental quality in the United States transportation sector amidst biomass-fossil energy consumption and growth. J. Clean. Prod. 2021, 285, 124863. [Google Scholar] [CrossRef]
- Al-mulali, U. Exploring the bi-directional long run relationship between energy consumption and life quality. Renew. Sustain. Energy Rev. 2016, 54, 824–837. [Google Scholar] [CrossRef]
- Friedmann, S.J.; Fan, Z.; Tang, K. Low-Carbon Heat Solutions for Heavy Industry: Sources, Options, and Costs Today; CGEP, Columbia University: New York, NY, USA, 2019. [Google Scholar]
- He, K.; Wang, L. A review of energy use and energy-efficient technologies for the iron and steel industry. Renew. Sustain. Energy Rev. 2017, 70, 1022–1039. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, J.; Zhang, Z.; Manovic, V. Inherent potential of steelmaking to contribute to decarbonization targets via industrial carbon capture and storage. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ryberg, M.; Yang, Y.; Feng, K.; Kara, S.; Hauschild, M.; Chen, W.-Q. Efficiency stagnation in global steel production urges joint supply- and demand-side mitigation efforts. Nat. Commun. 2021, 12, 2066–2076. [Google Scholar] [CrossRef] [PubMed]
- Naito, M.; Takeda, K.; Matsui, Y. Ironmaking technology for the last 100 years: Deployment to advanced technologies from introduction of technological know-how, and evolution to next-generation process. ISIJ Int. 2015, 55, 7–35. [Google Scholar] [CrossRef] [Green Version]
- Uribe-Soto, W.; Portha, J.-F.; Commenge, J.-M.; Falk, L. A review of thermochemical processes and technologies to use steelworks off-gases. Renew. Sustain. Energy Rev. 2017, 74, 809–823. [Google Scholar] [CrossRef]
- Park, J.E.; Koo, K.Y.; Jung, U.H.; Lee, J.H.; Roh, H.-S.; Yoon, W.L. Syngas production by combined steam and CO2 reforming of coke oven gas over highly sinter-stable La-promoted Ni/MgAl2O4 catalyst. Int. J. Hydrogen Energy 2015, 40, 13909–13917. [Google Scholar] [CrossRef]
- Aghaali, M.H.; Firoozi, S. Enhancing the catalytic performance of Co substituted NiAl2O4 spinel by ultrasonic spray pyrolysis method for steam and dry reforming of methane. Int. J. Hydrogen Energy 2021, 46, 357–373. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Kim, B.-J.; Park, H.-R.; Ahn, S.-Y.; Kim, K.-J.; Roh, H.-S. Customized Ni–MgO–Al2O3 catalyst for carbon dioxide reforming of coke oven gas: Optimization of preparation method and co-precipitation pH. J. CO2 Util. 2020, 42, 101354–101362. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, B.; Wang, Z.; Kawi, S. High carbon resistant Ni@Ni phyllosilicate@SiO2 core shell hollow sphere catalysts for low temperature CH4 dry reforming. J. CO2 Util. 2018, 27, 238–246. [Google Scholar] [CrossRef]
- Karam, L.; Miglio, A.; Specchia, S.; Hassan, N.E.; Massiani, P.; Reboul, J. PET waste as organic linker source for the sustainable preparation of MOF-derived methane dry reforming catalysts. Mater. Adv. 2021, 2, 2750–2758. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Zhang, X.; Wang, Z.; Wang, X.; Peng, H. Design of Ni-ZrO2@SiO2 catalyst with ultra-high sintering and coking resistance for dry reforming of methane to prepare syngas. J. CO2 Util. 2018, 27, 297–307. [Google Scholar] [CrossRef]
- Jang, W.J.; Shim, J.O.; Kim, H.M.; Yoo, S.Y.; Roh, H.S. A review on dry reforming of methane in aspect of catalytic properties. Catal Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Tian, H.; Zeng, L.; Zhao, Z.J.; Gong, J. Dry reforming of methane over Ni/La2O3 nanorod catalysts with stabilized Ni nanoparticles. Appl. Catal. B Environ. 2017, 202, 683–694. [Google Scholar] [CrossRef]
- Li, G.; Cheng, H.; Zhao, H.; Lu, X.; Xu, Q.; Wu, C. Hydrogen production by CO2 reforming of CH4 in coke oven gas over Ni–Co/MgAl2O4 catalysts. Catal Today 2018, 318, 46–51. [Google Scholar] [CrossRef]
- Jang, W.J.; Kim, H.M.; Shim, J.O.; Yoo, S.Y.; Jeon, K.W.; Na, H.S.; Lee, Y.L.; Jeong, D.W.; Bae, J.W.; Nah, I.W.; et al. Key properties of Ni–MgO–CeO2, Ni–MgO–ZrO2, and Ni–MgO–Ce(1−x)Zr(x)O2 catalysts for the reforming of methane with carbon dioxide. Green Chem. 2018, 20, 1621–1633. [Google Scholar] [CrossRef]
- Ren, H.-P.; Ding, S.-Y.; Ma, Q.; Song, W.-Q.; Zhao, Y.-Z.; Liu, J.; He, Y.-M.; Tian, S.-P. The Effect of Preparation Method of Ni-Supported SiO2 Catalysts for Carbon Dioxide Reforming of Methane. Catalysts 2021, 11, 1221. [Google Scholar] [CrossRef]
- Talkhoncheh, S.K.; Haghighi, M. Syngas production via dry reforming of methane over Ni-based nanocatalyst over various supports of clinoptilolite, ceria and alumina. J. Nat. Gas Sci. Eng. 2015, 23, 16–25. [Google Scholar] [CrossRef]
- Mozammel, T.; Dumbre, D.; Hubesch, R.; Yadav, G.D.; Selvakannan, P.R.; Bhargava, S.K. Carbon Dioxide Reforming of Methane over Mesoporous Alumina Supported Ni(Co), Ni(Rh) Bimetallic, and Ni(CoRh) Trimetallic Catalysts: Role of Nanoalloying in Imoriving the Stability and Nature of Coking. Energy Fuels 2020, 34, 16433–16444. [Google Scholar] [CrossRef]
- Jang, W.J.; Jeong, D.W.; Shim, J.O.; Kim, H.M.; Han, W.B.; Bae, J.W.; Roh, H.S. Metal oxide (MgO, CaO, and La2O3) promoted Ni-Ce0.8Zr0.2O2 catalysts for H2 and CO production from two major greenhouse gases. Renew. Energy 2015, 79, 91–95. [Google Scholar] [CrossRef]
- Han, J.W.; Park, J.S.; Choi, M.S.; Lee, H.J. Uncoupling the size and support effects of Ni catalysts for dry reforming of methane. Appl. Catal. B Environ. 2017, 203, 625–632. [Google Scholar] [CrossRef]
- Margossian, T.; Larmier, K.; Kim, S.M.; Krumeich, F.; Fedorov, A.; Chen, P.; Müller, C.R.; Copéret, C. Molucularly Tailored Nickel Precursor and Support Yield a Stable Methane Dry Reforming Catalyst with Superior Metal Utilization. J. Am. Chem. Soc. 2017, 139, 6919–6927. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Huo, M.; Li, L.; Lyu, S.; Wang, J.; Wang, X.; Zhang, Y.; Li, J. Effect of alumina morphology on dry reforming of methane over Ni/Al2O3 catalysts. Catal. Sci. Technol. 2020, 10, 510–516. [Google Scholar] [CrossRef]
- Shamskar, F.R.; Rezaei, M.; Meshkani, F. The influence of Ni loading on the activity and coke formation of ultrasound-assisted co-precipitated Ni–Al2O3 nanocatalyst in dry reforming of methane. Int. J. Hydrogen Energy 2017, 42, 4155–4164. [Google Scholar] [CrossRef] [Green Version]
- Hou, Z.; Yokota, O.; Tanaka, T.; Yashima, T. Characterization of Ca-promoted Ni/-Al2O3 catalyst for CH4 reforming with CO2. Appl. Catal. A Gen. 2003, 253, 381–387. [Google Scholar] [CrossRef]
- Alipoura, Z.; Rezaei, M.; Meshkani, F. Effect of Ni loadings on the activity and coke formation of MgO-modified Ni/Al2O3 nanocatalyst in dry reforming of methane. J. Energy Chem. 2014, 23, 633–638. [Google Scholar] [CrossRef]
- Sengupta, S.; Deo, G. Modifying alumina with CaO or MgO in supported Ni and Ni–Co catalysts and its effect on dry reforming of CH4. J. CO2 Util. 2015, 10, 67–77. [Google Scholar] [CrossRef]
- Guo, J.; Lou, H.; Zheng, X. The deposition of coke from methane on a Ni/MgAl2O4 catalyst. Carbon 2007, 45, 1314–1321. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Qiu, C.T.; Xu, H.D.; Lin, T.; Lin, Z.E.; Gong, M.C. Low-temperature selective catalytic reduction of NO with NH3 over monolith catalyst of MnOx/CeO2-ZrO2-Al2O3. Catal. Today 2011, 175, 171–176. [Google Scholar] [CrossRef]
- Ko, J.H.; Park, S.H.; Jeon, J.K.; Kim, S.S.; Kim, S.C.; Kim, J.M.; Chang, D.; Park, Y.K. Low temperature selective catalytic reduction of NO with NH3 over Mn supported on Ce0.65Zr0.35O2 prepared by supercritical method: Effect of Mn precursors on NO reduction. Catal. Today 2012, 185, 290–295. [Google Scholar] [CrossRef]
- Shen, B.; Zhang, X.; Ma, H.; Yao, Y.; Liu, T. A comparative study of Mn/CeO2, Mn/ZrO2 and Mn/Ce-ZrO2 for low temperature selective catalytic reduction of NO with NH3 in the presence of SO2 and H2O. J. Environ. Sci. 2013, 25, 791–800. [Google Scholar] [CrossRef]
- Rynkowski, J.M.; Paryjczak, T.; Lenik, M. On the nature of oxidic nickel phases in NiO/y-Al2O3 catalysts. Appl. Catal. A 1993, 106, 73–82. [Google Scholar] [CrossRef]
- Salagre, P.; Fierro, J.L.G.; Medina, F.; Sueiras, J.E. Characterization of nickel species on several y-alumina supported nickel sample. J. Mol. Catal. A 1996, 106, 125–134. [Google Scholar] [CrossRef]
- Turlier, P.; Praliaud, H.; Moral, P.; Martin, G.A.; Dalmon, J.A. Influence of the nature of the support on the reducibility and catalytic properties of nickel: Evidence for a new type of metal support interaction. Appl. Catal. 1985, 19, 287–300. [Google Scholar] [CrossRef]
- Xu, D.; Li, W.; Ge, Q.; Xu, H. A novel process for converting coalmine-drained methane gas to syngas over nickel–magnesia solid solution catalyst. Fuel Process. Technol. 2005, 86, 995–1006. [Google Scholar] [CrossRef]
- Arena, F.; Frusteri, F.; Giordano, N. Temperature-programmed Reduction Study of NiO-MgO Interactions in Magnesia-supported Ni Catalysts and NiO-MgO Physical Mixture. J. Chem. Soc. Faraday Trans. 1990, 86, 2663–2669. [Google Scholar] [CrossRef]
- Wang, Y.; Ohtsuka, Y. Mn-based binary oxides as catalysts for the conversion of methane to C2 hydrocarbons with carbon dioxide as oxidant. Appl. Catal. A 2001, 219, 183–193. [Google Scholar] [CrossRef]
- Zangeneh, F.T.; Sahebdelfar, S.; Ravanchi, T.M. Conversion of carbon dioxide to valuable petrochemicals: An approach to clean development mechanism. J. Nat. Gas Chem. 2011, 20, 219–231. [Google Scholar] [CrossRef]
- Roh, H.-S.; Potdar, H.S.; Jun, K.-W.; Kim, J.-W.; Oh, Y.-S. Carbon dioxide reforming of methane over Ni incorporated into Ce–ZrO2 catalysts. Appl. Catal. A 2004, 276, 231–239. [Google Scholar] [CrossRef]








| Catalyst | Support BET S.A. (m2/g) 1 | Catalyst BET S.A. (m2/g) 1 | Ni Dispersion (%) 2 | Crystallite Size of Ni0 (nm) 3 |
|---|---|---|---|---|
| Ni/MG10 | 202.6 | 139.2 | 1.46 | 13.6 |
| Ni/MG30 | 200.9 | 120.8 | 3.41 | 9.4 |
| Ni/MG50 | 164.2 | 104.2 | 3.32 | 9.7 |
| Ni/MG70 | 161.5 | 104.0 | 3.18 | 9.9 |
| Ni/MG90 | 102.0 | 76.6 | 3.24 | 11.9 |
| Catalyst | Ni/MG10 | Ni/MG30 | Ni/MG50 | Ni/MG70 | Ni/MG90 |
|---|---|---|---|---|---|
| Reduction degree (%) 1 | 84 | 96 | 88 | 67 | 51 |
| Catalyst | Support Desorbed CO2 (cm3/gcat) | Total Desorbed CO2 (cm3/gcat) |
|---|---|---|
| Ni/MG10 | 18.0 | 6.1 |
| Ni/MG30 | 58.8 | 9.7 |
| Ni/MG50 | 71.2 | 8.2 |
| Ni/MG70 | 154.3 | 8.0 |
| Ni/MG90 | 39.8 | 7.7 |
| Gas Composition (%) 1 | ||||||
|---|---|---|---|---|---|---|
| Component | CH4 | CO2 | N2 | H2 | CO | Total |
| Reactant gas (COG) + CO2 | 21.39 | 25.67 | 4.46 | 42.39 | 6.10 | 100.00 |
| Product gas | 2.65 | 2.68 | 3.53 | 55.27 | 35.87 | 100.00 |
| Yield (%) | Conversion (%) | H2/CO ratio | ||||
| H2 | CO | CH4 | CO2 | Reactant gas (COG) + CO2 | Product gas | |
| 64.0 | 83.2 | 84.4 | 86.8 | 6.95 | 1.54 | |
| MgO:Al2O3 | Name |
|---|---|
| 1:9 | Ni/MG10 |
| 3:7 | Ni/MG30 |
| 5:5 | Ni/MG50 |
| 7:3 | Ni/MG70 |
| 9:1 | Ni/MG90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-R.; Kim, B.-J.; Lee, Y.-L.; Ahn, S.-Y.; Kim, K.-J.; Hong, G.-R.; Yun, S.-J.; Jeon, B.-H.; Bae, J.W.; Roh, H.-S. CO2 Reforming of CH4 Using Coke Oven Gas over Ni/MgO-Al2O3 Catalysts: Effect of the MgO:Al2O3 Ratio. Catalysts 2021, 11, 1468. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11121468
Park H-R, Kim B-J, Lee Y-L, Ahn S-Y, Kim K-J, Hong G-R, Yun S-J, Jeon B-H, Bae JW, Roh H-S. CO2 Reforming of CH4 Using Coke Oven Gas over Ni/MgO-Al2O3 Catalysts: Effect of the MgO:Al2O3 Ratio. Catalysts. 2021; 11(12):1468. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11121468
Chicago/Turabian StylePark, Ho-Ryong, Beom-Jun Kim, Yeol-Lim Lee, Seon-Yong Ahn, Kyoung-Jin Kim, Ga-Ram Hong, Seong-Jin Yun, Byong-Hun Jeon, Jong Wook Bae, and Hyun-Seog Roh. 2021. "CO2 Reforming of CH4 Using Coke Oven Gas over Ni/MgO-Al2O3 Catalysts: Effect of the MgO:Al2O3 Ratio" Catalysts 11, no. 12: 1468. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11121468