Enhanced Hydrocarbons Biodegradation at Deep-Sea Hydrostatic Pressure with Microbial Electrochemical Snorkels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Bioelectrochemical Snorkels Enhance Alkanes Biodegradation at Deep-Sea and Ambient HP
2.2. Bioelectrochemical Snorkels Regenerate SO42− in Sediments at Deep-Sea and Ambient HP
3. Materials and Methods
3.1. Sediment Sampling
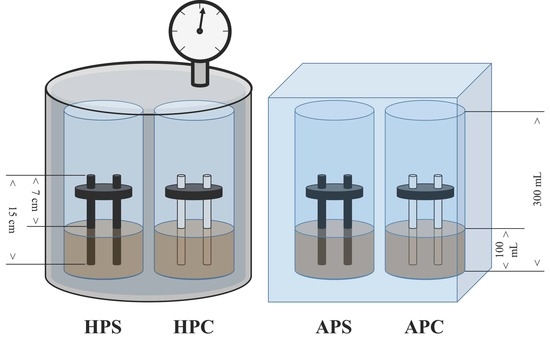
3.2. Reactors Configuration
3.3. Sampling Procedure
3.4. Analytical Procedures
3.5. Microsensor Measurements
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meckenstock, R.U.; Boll, M.; Mouttaki, H.; Koelschbach, J.S.; Cunha Tarouco, P.; Weyrauch, P.; Dong, X.; Himmelberg, A.M. Anaerobic Degradation of Benzene and Polycyclic Aromatic Hydrocarbons. J. Mol. Microbiol. Biotechnol. 2016, 26, 92–118. [Google Scholar] [CrossRef]
- Meckenstock, R.U.; Elsner, M.; Griebler, C.; Lueders, T.; Stumpp, C.; Aamand, J.; Agathos, S.N.; Albrechtsen, H.J.; Bastiaens, L.; Bjerg, P.L.; et al. Biodegradation: Updating the concepts of control for microbial cleanup in contaminated aquifers. Environ. Sci. Technol. 2015, 49, 7073–7081. [Google Scholar] [CrossRef] [Green Version]
- White, C.; Gadd, G.M. Mixed sulphate-reducing bacterial cultures for bioprecipitation of toxic metals: Factorial and response-surface analysis of the effects of dilution rate, sulphate and substrate concentration. Microbiology 1996, 142, 2197–2205. [Google Scholar] [CrossRef] [Green Version]
- O’Flaherty, V.; Mahony, T.; O’Kennedy, R.; Colleran, E. Effect of pH on growth kinetics and sulphide toxicity thresholds of a range of methanogenic, syntrophic and sulphate-reducing bacteria. Process Biochem. 1998, 33, 555–569. [Google Scholar] [CrossRef]
- Rabaey, K.; Rodriguez, J.; Blackall, L.L.; Keller, J.; Gross, P.; Batstone, D.; Verstraete, W.; Nealson, K.H. Microbial ecology meets electrochemistry: Electricity-driven and driving communities. ISME J. 2007, 1, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Gannon, S.M.; Nevin, K.P.; Franks, A.E.; Lovley, D.R. Stimulating the anaerobic degradation of aromatic hydrocarbons in contaminated sediments by providing an electrode as the electron acceptor. Environ. Microbiol. 2010, 12, 1011–1020. [Google Scholar] [CrossRef]
- Rakoczy, J.; Feisthauer, S.; Wasmund, K.; Bombach, P.; Neu, T.R.; Vogt, C.; Richnow, H.H. Benzene and sulfide removal from groundwater treated in a microbial fuel cell. Biotechnol. Bioeng. 2013, 110, 3104–3113. [Google Scholar] [CrossRef]
- Wei, M.; Harnisch, F.; Vogt, C.; Ahlheim, J.; Neu, T.R.; Richnow, H.H. Harvesting electricity from benzene and ammonium-contaminated groundwater using a microbial fuel cell with an aerated cathode. RSC Adv. 2015, 5, 5321–5330. [Google Scholar] [CrossRef]
- Luo, H.P.; Liu, G.L.; Zhang, R.D.; Jin, S. Phenol degradation in microbial fuel cells. Chem. Eng. J. 2009, 147, 259–264. [Google Scholar] [CrossRef]
- Feng, C.H.; Huang, L.Q.; Yu, H.; Yi, X.Y.; Wei, C.H. Simultaneous phenol removal, nitrification and denitrification using microbial fuel cell technology. Water Res. 2015, 76, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Hedbavna, P.; Rolfe, S.A.; Huang, W.E.; Thornton, S.F. Biodegradation of phenolic compounds and their metabolites in contaminated groundwater using microbial fuel cells. Bioresour. Technol. 2016, 200, 426–434. [Google Scholar] [CrossRef]
- Daghio, M.; Vaiopoulou, E.; Patil, S.A.; Suarez-Suarez, A.; Head, I.M.; Franzetti, A.; Rabaey, K. Anodes Stimulate Anaerobic Toluene Degradation via Sulfur Cycling in Marine Sediments. Appl. Environ. Microbiol. 2016, 82, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Sherafatmand, M.; Ng, H.Y. Using sediment microbial fuel cells (SMFCs) for bioremediation of polycyclic aromatic hydrocarbons (PAHs). Bioresour. Technol. 2015, 195, 122–130. [Google Scholar] [CrossRef]
- Viggi, C.C.; Presta, E.; Bellagamba, M.; Kaciulis, S.; Balijepalli, S.K.; Zanaroli, G.; Petrangeli Papini, M.; Rossetti, S.; Aulenta, F. The “Oil-Spill Snorkel”: An innovative bioelectrochemical approach to accelerate hydrocarbons biodegradation in marine sediments. Front. Microbiol. 2015, 6, 881. [Google Scholar]
- Viggi, C.C.; Matturro, B.; Frascadore, E.; Insogna, S.; Mezzi, A.; Kaciulis, S.; Sherry, A.; Mejeha, O.K.; Head, I.M.; Vaiopoulou, E.; et al. Bridging spatially segregated redox zones with a microbial electrochemical snorkel triggers biogeochemical cycles in oil-contaminated River Tyne (UK) sediments. Water Res. 2017, 127, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzocchi, U.; Palma, E.; Rossetti, S.; Aulenta, F.; Scoma, A. Parallel artificial and biological electric circuits power petroleum decontamination: The case of snorkel and cable bacteria. Water Res. 2020, 173, 115520. [Google Scholar] [CrossRef]
- Scoma, A.; Yakimov, M.M.; Boon, N. Challenging Oil Bioremediation at Deep-Sea Hydrostatic Pressure. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Jernelöv, A. How to defend against future oil spills. Nature 2010, 466, 182–183. [Google Scholar] [CrossRef] [PubMed]
- The Federal Interagency Solutions Group, Oil Budget Calculator Science and Engineering Team. Oil Budget Calculator (Deepwater Horizon); Technical Documentation; The Federal Interagency Solutions Group, Oil Budget Calculator Science and Engineering Team: Washington, DC, USA, 2010. [Google Scholar]
- NOAA/Hazardous Materials Response and Assessment Division Seattle, Washington. Oil Spill Case Histories; NOAA/Hazardous Materials Response and Assessment Division Seattle, Washington: Silver Spring, MD, USA, 1992; pp. 1–224. [Google Scholar]
- Michel, J.; Gilbert, T.; Etkin, D.S.; Urban, R.; Waldron, J.; Blocksidge, C.T. Potentially Polluting Wrecks in Marine Waters. In Proceedings of the 2005 International Oil Spill Conference, Miami Beach, FL, USA, 15–19 May 2005; pp. 1–38. [Google Scholar]
- Mugge, R.; Brock, M.; Salerno, J.L.; Damour, M.; Church, R.A.; Lee, J.S.; Hamdan, L.J. Deep-Sea Biofilms, Historic Shipwreck Preservation and the Deepwater Horizon Spill. Front. Mar. Sci. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Hoareau, M.; Erable, B.; Bergel, A. Microbial electrochemical snorkels (MESs): A budding technology for multiple applications. A mini review. Electrochem. Comm. 2019, 104, 106473. [Google Scholar] [CrossRef]
- Mitov, M.; Bardarov, I.; Chorbadzhiyska, E.; Kostov, K.L.; Hubenova, Y. First evidence for applicability of the microbial electrochemical snorkel for metal recovery. Electrochem. Comm. 2021, 122, 106889. [Google Scholar] [CrossRef]
- Reimers, C.E.; Girguis, P.; Stecher, H.A.; Tender, L.M.; Ryckelynck, N.; Whaling, P. Microbial fuel cell energy from an ocean cold seep. Geobiology 2006, 4, 123–136. [Google Scholar] [CrossRef]
- Nielsen, M.E.; Reimers, C.E.; White, H.K.; Sharma, S.; Girguis, P.R. Sustainable energy from deep ocean cold seeps. Energy Environ. Sci. 2008, 1, 584–593. [Google Scholar] [CrossRef]
- Kobayashi, H.; Nagashima, A.; Kouyama, M.; Fu, Q.; Ikarashi, M.; Maeda, H.; Sato, K. High-pressure thermophilic electromethanogenic system producing methane at 5 MPa, 55 °C. J. Biosci. Bioeng. 2017, 124, 327–332. [Google Scholar] [CrossRef]
- Scoma, A.; Heyer, R.; Rifai, R.; Dandyk, C.; Marshall, I.; Kerckhof, F.M.; Marietou, A.; Boshker, H.T.S.; Meysman, F.J.R.; Malmos, K.G.; et al. Reduced TCA cycle rates at high hydrostatic pressure hinder hydrocarbon degradation and obligate oil degraders in natural, deep-sea microbial communities. ISME J. 2018, 13, 1004–1018. [Google Scholar] [CrossRef]
- Tamburini, C.; Boutrif, M.; Garel, M.; Colwell, R.R.; Deming, J.W. Prokaryotic responses to hydrostatic pressure in the ocean—A review. Environ. Microbiol. 2013, 15, 1262–1274. [Google Scholar] [CrossRef] [PubMed]
- Deming, J. Bacterial growth in deep-sea sediment trap and boxcore samples. Mar. Ecol. Prog. Ser. 1985, 25, 305–312. [Google Scholar] [CrossRef]
- Schwarz, J.R.; Walker, J.D.; Colwell, R.R. Deep-Sea Bacteria: Growth and Utilization of Hydrocarbons at Ambient and In Situ Pressure. Appl. Microbiol. 1974, 28, 982–986. [Google Scholar] [CrossRef]
- Schwarz, J.R.; Walker, J.D.; Colwell, R.R. Deep-sea bacteria: Growth and utilization on n-hexadecane at in situ temperature and pressure. Can. J. Microbiol. 1975, 21, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Marietou, A.; Chastain, R.; Beulig, F.; Scoma, A.; Hazen, T.C.; Bartlett, D.H. The Effect of Hydrostatic Pressure on Enrichments of Hydrocarbon Degrading Microbes from the Gulf of Mexico Following the Deepwater Horizon Oil Spill. Front. Microbiol. 2018, 9, 808. [Google Scholar] [CrossRef]
- Nguyen, U.T.; Lincoln, S.A.; Valladares Juarez, A.G.; Schedler, M.; Macalady, J.L.; Muller, R.; Freeman, K.H. The influence of pressure on crude oil biodegradation in shallow and deep Gulf of Mexico sediments. PLoS ONE 2018, 13, e0199784. [Google Scholar] [CrossRef] [PubMed]
- Jannasch, H.W.; Taylor, C.D. Deep-sea Microbiology. Ann. Rev. Microbiol. 1984, 38, 487–514. [Google Scholar] [CrossRef]
- Yayanos, A.A.; Chastain, R. The influence of nutrition on the physiology of piezophilic bacteria. In Proceedings of the 8th International Symposium on Microbial Ecology, Halifax, NS, Canada, 9–14 August 1998; p. 6. [Google Scholar]
- Scoma, A.; Yakimov, M.M.; Daffonchio, D.; Boon, N. Self-healing capacity of deep-sea ecosystems affected by petroleum hydrocarbons: Understanding microbial oil degradation at hydrocarbon seeps is key to sustainable bioremediation protocols. EMBO Rep. 2017, 18, 868–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yayanos, A.A. Evolutional and ecological implications of the properties of deep-sea barophilic bacteria. Proc. Natl. Acad. Sci. USA 1986, 83, 9542–9546. [Google Scholar] [CrossRef] [Green Version]
- Jannasch, H.W.; Wirsen, C.O. Variability of pressure adaptation in deep sea bacteria. Arch. Microbiol. 1984, 139, 281–288. [Google Scholar] [CrossRef]
- Talley, L.; Pickard, G.; Emery, W.; Swift, J. (Eds.) Typical Distributions of Water Characteristics. In Descriptive Physical Oceanography; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; pp. 67–110. [Google Scholar]
- Dutta, P.K.; Keller, J.; Yuan, Z.G.; Rozendal, R.A.; Rabaey, K. Role of Sulfur during Acetate Oxidation in Biological Anodes. Environ. Sci. Technol. 2009, 43, 3839–3845. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Ebrahim, A.; Feist, A.M.; Embree, M.; Zhang, T.; Lovley, D.; Zengler, K. Sulfide-driven microbial electrosynthesis. Environ. Sci. Technol. 2013, 47, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Li, S.L.; Nealson, K.H. Enriching distinctive microbial communities from marine sediments via an electrochemical-sulfide-oxidizing process on carbon electrodes. Front. Microbiol. 2015, 6, 111. [Google Scholar] [CrossRef]
- Rabaey, K.; Clauwaert, P.; Aelterman, P.; Verstraete, W. Tubular microbial fuel cells for efficient electricity generation. Environ. Sci. Technol. 2005, 39, 8077–8082. [Google Scholar] [CrossRef] [PubMed]
- McDonough, K.M.; Dzombak, D.A. Microcosm Approach to Study Transport of Polychlorinated Biphenyls in Sediment. J. Environ. Eng. 2006, 132, 689–693. [Google Scholar] [CrossRef]
- Scoma, A.; Garrido-Amador, P.; Nielsen, S.D.; Roy, H.; Kjeldsen, K.U. The Polyextremophilic Bacterium Clostridium paradoxum Attains Piezophilic Traits by Modulating Its Energy Metabolism and Cell Membrane Composition. Appl. Environ. Microbiol. 2019, 85, e00802–e008019. [Google Scholar] [CrossRef] [Green Version]
- Revsbech, N.P.; Jorgensen, B.B. Microelectrodes—Their use in Microbial Ecology. Adv. Microb. Ecol. 1986, 9, 293–352. [Google Scholar]
- Revsbech, N.P. An Oxygen Microsensor with a Guard Cathode. Limnol. Oceanogr. 1989, 34, 474–478. [Google Scholar] [CrossRef]
- Jeroschewski, P.; Steuckart, C.; Kuhl, M. An amperometric microsensor for the determination of H2S in aquatic environments. Anal. Chem. 1996, 68, 4351–4357. [Google Scholar] [CrossRef]
- Marzocchi, U.; Bonaglia, S.; van de Velde, S.; Hall, P.O.J.; Schramm, A.; Risgaard-Petersen, N.; Meysman, F.J.R. Transient bottom water oxygenation creates a niche for cable bacteria in long-term anoxic sediments of the Eastern Gotland Basin. Environ. Microbiol. 2018, 20, 3031–3041. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Yu, H.Q. Stimulating sediment bioremediation with benthic microbial fuel cells. Biotechnol. Adv. 2015, 33, 1–12. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aulenta, F.; Palma, E.; Marzocchi, U.; Cruz Viggi, C.; Rossetti, S.; Scoma, A. Enhanced Hydrocarbons Biodegradation at Deep-Sea Hydrostatic Pressure with Microbial Electrochemical Snorkels. Catalysts 2021, 11, 263. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020263
Aulenta F, Palma E, Marzocchi U, Cruz Viggi C, Rossetti S, Scoma A. Enhanced Hydrocarbons Biodegradation at Deep-Sea Hydrostatic Pressure with Microbial Electrochemical Snorkels. Catalysts. 2021; 11(2):263. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020263
Chicago/Turabian StyleAulenta, Federico, Enza Palma, Ugo Marzocchi, Carolina Cruz Viggi, Simona Rossetti, and Alberto Scoma. 2021. "Enhanced Hydrocarbons Biodegradation at Deep-Sea Hydrostatic Pressure with Microbial Electrochemical Snorkels" Catalysts 11, no. 2: 263. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11020263