Advances in Valorization of Lignocellulosic Biomass towards Energy Generation
Abstract
:1. Introduction
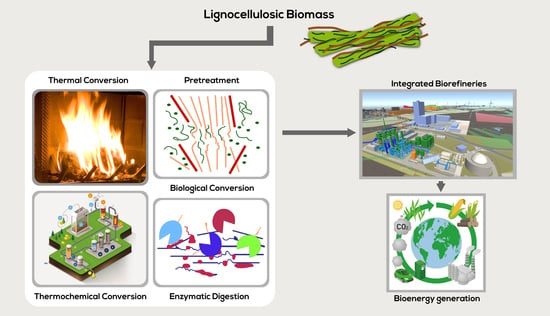
2. Lignocellulosic Biomass Structure
3. Routes of Lignocellulosic Conversion
3.1. Thermal Conversion
Combustion
3.2. Thermochemical Conversion
3.2.1. Gasification
| Gasification Type | Temperature Range/°C | Location of Industrial Plant | Type of Biomass | End Product | Functional Unit | References |
|---|---|---|---|---|---|---|
| Fixed bed | 700–800 | [32] | ||||
| Downdraft | Liaoning, China | Wood residues | Gas for cooking | 700 kWt | [33] | |
| Jilin, China | Agricultural residues | Power and heat | 200 kWe | |||
| Hunan, China | Crop residues | Gas for cooking | 300 kWt | |||
| Updraft | Vilhelmina, Sweden | Wood chip | Heat | 4–6 MWth | [34] | |
| Harboøre, Denmark | Wood chip | Heat and power | 4 MWth | |||
| Ankara, Turkey | Wood chip | Heat and power | 2 MWe | |||
| Fluidized bed | 1000 °C | [32] | ||||
| Circulating fluidized bed | Värnamo, Sweden | Wood chips | Heat and power | 6 MWe/9 MWth | [35] | |
| Ruien, Belgium | Woodchip | Syngas used to power Pulverised Coal (PC) boilers | 50 MWth | [34] | ||
| Varkaus, Finland | wood | Biomass to liquid fuel (diesel), Syngas used for lime kiln | 50 Mwth | |||
| Lahti, Finland | Forest residues, sawdust, short rotation forest (SRF), and bark | Syngas used to power PC boilers | 45 MWth | |||
| Vaskiluodon Voima Oy, Finland | Forest residue | Co-firing | 140 MWth | |||
| Bubbling fluidized bed | Jiangsu, China | Rice husk | Heat | 120 MWth | [33] | |
| Anhui, China | Rice husk | Electricity | 400 kWe | |||
| Dual fluidized bed | Oberwart, Austria | Wood chip | Heat and power | 10 MWth | [34] | |
| Güssing, Austria | Wood chip | Heat and power | 8 MWth | |||
| Entrained | 1400–1500 °C | [32] | ||||
| Freiberg, Germany | Wood | Biomass to liquid fuel | 45 MWth | [34] | ||
3.2.2. Pyrolysis
3.3. Biological Conversion
3.3.1. Direct Conversion
3.3.2. Indirect Conversion
Pretreatment Technologies
Physical Methods
| Types of Extrusion Methods Used in Combination | Description of the Process | References |
|---|---|---|
| Steam explosion extrusion | High pressure and high steam temperature are applied to treat the biomass for a short time and then this pressure is released by extrusion. This results in the expansion of steam that provides a shear force for the disruption of the cell wall of the plant cell. | [54] |
| Acid extrusion | Pretreatment of biomass is done by dilute acid and then this is added to the hot water extraction system. | [55] |
| Alkaline extrusion | Biomass is soaked in NaOH solution and added to the extruder with the help of a volumetric pump. The alkali acts as a delignification agent and prevents carbohydrate degradation. | [56] |
| Vacuum extrusion | Biomass is treated in vacuum extrusion. | [57] |
| Ammonia fiber explosion and extrusion | Biomass is extruded with ammonia that aids in expanding the fiber. | [58] |
| Extrusion and heat moisture treatment | Heat moisture treatment and extrusion are used in combination to pretreat the biomass with physicochemical properties. | [59] |
Milling
Sonication
Pulsed Electric Field (PEF)
Chemical Methods
Acid Pretreatment
Ionic Liquid Pretreatment
Organic Solvent Pretreatment
Ozonolysis/Plasma
Physio-Chemical Methods
Hot Water Pretreatment
Supercritical CO2 Explosion
Ammonia Fiber Expansion (AFEX)
Microwave Pretreatment
Wet Oxidation
Biological Methods
| Pretreatment | Limitations | Sorted Examples of Recent Studies of Pretreatment Technologies | References | ||
|---|---|---|---|---|---|
| Inhibitor production | Process Cost * | Feedstock | Results | ||
| Physical Methods | |||||
| Mechanicalextrusion | Low | High |
|
| [93,94] |
| Milling | Low | Low |
|
| [63,91,95,96] |
| Sonication | Low | High |
|
| [94,97,98] |
| Pulsed electric field (PEF) | Low | Low |
|
| [99] |
| Chemical Methods | |||||
| Alkaline pretreatment | Low | High |
|
| [94,100,101,102] |
| Acidic pretreatment | High | High |
|
| [78,103,104] |
| Ionic liquid | Low | High |
|
| [94,105] |
| Organic solvent | High | High |
|
| [63,106] |
| Ozonolysis | Low | High |
|
| [107] |
| Physiochemical Methods | |||||
| Steam explosion | High | High |
|
| [94,108,109] |
| Hot water pretreatment | Low | Low |
|
| [94,110] |
| Supercritical CO2 explosion | Low | High |
|
| [63,111] |
| Ammonia fiber expansion (AFEX) | Low | Low |
|
| [63,112] |
| Microwave pretreatment | Low | High |
|
| [94,113] |
| Wet oxidation | Low | High |
|
| [114] |
| Biological methods | Low | Low |
|
| [115,116] |
Enzyme Digestibility
4. Integrated Biorefineries for Lignocellulosic Biomass
5. Future Prospects and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan Cheah, W.; Sankaran, R.; Loke Show, P.; Nilam Baizura Tg Ibrahim, T.; Wayne Chew, K.; Culaba, A.; Chang, J.-S. Pretreatment Methods for Lignocellulosic Biofuels Production: Current Advances, Challenges and Future Prospects. Biofuel Res. J. 2020, 25, 1115–1127. [Google Scholar] [CrossRef] [Green Version]
- Den, W.; Sharma, V.K.; Lee, M.; Nadadur, G.; Varma, R.S. Lignocellulosic Biomass Transformations via Greener Oxidative Pretreatment Processes: Access to Energy and Value Added Chemicals. Front. Chem. 2018, 6, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated Lignocellulosic Value Chains in a Growing Bioeconomy: Status Quo and Perspectives. GCB Bioenergy 2018, 11, gcbb.12586. [Google Scholar] [CrossRef] [Green Version]
- Mapemba, L.; Epplin, F.M. Lignocellulosic Biomass Harvest and Delivery Cost; Annual Meeting; 1364-2016-108017; Southern Agricultural Economics Association: Tulsa, Oklahoma, 14–18 February 2004. [Google Scholar]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Biochemical Production: A Review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef] [Green Version]
- Hamaguchi, M.; Kautto, J.; Vakkilainen, E. Effects of Hemicellulose Extraction on the Kraft Pulp Mill Operation and Energy Use: Review and Case Study with Lignin Removal. Chem. Eng. Res. Des. 2013, 91, 1284–1291. [Google Scholar] [CrossRef]
- Samuel Dahunsi, O.; Enyinnaya, M. The Bioenergy Potentials of Lignocelluloses. In Energy Conversion—Current Technologies and Future Trends; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Nanda, S.; Mohammad, J.; Reddy, S.N.; Kozinski, J.A.; Dalai, A.K. Pathways of Lignocellulosic Biomass Conversion to Renewable Fuels. Biomass Convers. Biorefin. 2014, 4, 157–191. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Cho, J.; Talaiekhozani, A.; Sabbagh, F.; Hashemi, B.; Rupani, P.F.; Mohammadi, A.A. Different Pretreatment Technologies of Lignocellulosic Biomass for Bioethanol Production: An Overview. Energy 2020, 199, 117457. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Chen, H.; Abeln, F.; Auta, H.; Fan, J.; Budarin, V.L.; Clark, J.H.; Parsons, S.; Chuck, C.J.; Zhang, S.; et al. Chemicals from Lignocellulosic Biomass: A Critical Comparison between Biochemical, Microwave and Thermochemical Conversion Methods. Crit. Rev. Environ. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Özdenkçi, K.; De Blasio, C.; Muddassar, H.R.; Melin, K.; Oinas, P.; Koskinen, J.; Sarwar, G.; Järvinen, M. A Novel Biorefinery Integration Concept for Lignocellulosic Biomass. Energy Convers. Manag. 2017, 149, 974–987. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Li, C.; Dai, L.; Xu, C.; Zhong, Y.; Yu, F.; Si, C. Biomass Fractionation and Lignin Fractionation towards Lignin Valorization. ChemSusChem 2020, 4284–4295. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The Critical Role of Lignin in Lignocellulosic Biomass Conversion and Recent Pretreatment Strategies: A Comprehensive Review. Bioresour. Technol. 2020, 122784. [Google Scholar] [CrossRef]
- Ubando, A.T.; Rivera, D.R.T.; Chen, W.H.; Culaba, A.B. A Comprehensive Review of Life Cycle Assessment (LCA) of Microalgal and Lignocellulosic Bioenergy Products from Thermochemical Processes. Bioresour. Technol. 2019, 121837. [Google Scholar] [CrossRef]
- Islam, M.K.; Wang, H.; Rehman, S.; Dong, C.; Hsu, H.Y.; Lin, C.S.K.; Leu, S.Y. Sustainability Metrics of Pretreatment Processes in a Waste Derived Lignocellulosic Biomass Biorefinery. Bioresour. Technol. 2020, 122558. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Rajesh Banu, J.; Rao, C.V.; Kim, Y.G.; Yang, Y.H. Recent Developments in Pretreatment Technologies on Lignocellulosic Biomass: Effect of Key Parameters, Technological Improvements, and Challenges. Bioresour. Technol. 2020, 122724. [Google Scholar] [CrossRef] [PubMed]
- Beig, B.; Riaz, M.; Raza Naqvi, S.; Hassan, M.; Zheng, Z.; Karimi, K.; Pugazhendhi, A.; Atabani, A.E.; Thuy Lan Chi, N. Current Challenges and Innovative Developments in Pretreatment of Lignocellulosic Residues for Biofuel Production: A Review. Fuel 2020, 287, 119670. [Google Scholar] [CrossRef]
- Wang, F.; Ouyang, D.; Zhou, Z.; Page, S.J.; Liu, D.; Zhao, X. Lignocellulosic Biomass as Sustainable Feedstock and Materials for Power Generation and Energy Storage. J. Energy Chem. 2021, 57, 247–280. [Google Scholar] [CrossRef]
- Kumar, G.; Dharmaraja, J.; Arvindnarayan, S.; Shoban, S.; Bakonyi, P.; Saratale, G.D.; Nemestóthy, N.; Bélafi-Bakó, K.; Yoon, J.J.; Kim, S.H. A Comprehensive Review on Thermochemical, Biological, Biochemical and Hybrid Conversion Methods of Bio-Derived Lignocellulosic Molecules into Renewable Fuels. Fuel 2019, 251, 352–367. [Google Scholar] [CrossRef]
- Seidl, P.R.; Goulart, A.K. Pretreatment Processes for Lignocellulosic Biomass Conversion to Biofuels and Bioproducts. Curr. Opin. Green Sust. Chem. 2016, 2, 48–53. [Google Scholar] [CrossRef]
- Abraham, A.; Mathew, A.K.; Park, H.; Choi, O.; Sindhu, R.; Parameswaran, B.; Pandey, A.; Park, J.H.; Sang, B.I. Pretreatment Strategies for Enhanced Biogas Production from Lignocellulosic Biomass. Bioresour. Technol. 2020, 301, 122725. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.P.; Nguyen, L.N.; Vu, M.T.; Johir, M.A.H.; McLaughlan, R.; Nghiem, L.D. A Comprehensive Review on the Framework to Valorise Lignocellulosic Biomass as Biorefinery Feedstocks. Sci. Total Environ. 2020, 743, 140630. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.V.; Hamid, S.B.A.; Zain, S.K. Conversion of Lignocellulosic Biomass to Nanocellulose: Structure and Chemical Process. Sci. World J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Brown, R. Thermochemical Processing of Biomass: Conversion into Fuels, Chemicals and Power; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Vicente, E.D.; Vicente, A.M.; Evtyugina, M.; Tarelho, L.A.C.; Almeida, S.M.; Alves, C. Emissions from Residential Combustion of Certified and Uncertified Pellets. Renew. Energy 2020, 161, 1059–1071. [Google Scholar] [CrossRef]
- Das, S.; Basumatary, S.; Kalita, P.; Kulkarni, V.; Goswami, P.; Garg, A.; Peng, X. Bioelectricity Production from Lignocellulosic Biomass. In Lignocellulosic Biorefining Technologies; Wiley: Hoboken, NJ, USA, 2020; pp. 87–123. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of First and Second Generation Biofuels: A Comprehensive Review. Renew. Sust. Energ. Rev. 2010. [Google Scholar] [CrossRef]
- Patel, M.; Zhang, X.; Kumar, A. Techno-Economic and Life Cycle Assessment on Lignocellulosic Biomass Thermochemical Conversion Technologies: A Review. Renew. Sust. Energy Rev. 2016, 53, 1486–1499. [Google Scholar] [CrossRef]
- Puig-Arnavat, M.; Thomsen, T.P.; Ravenni, G.; Clausen, L.R.; Sárossy, Z.; Ahrenfeldt, J. Pyrolysis and Gasification of Lignocellulosic Biomass. In Biorefinery; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 79–110. [Google Scholar] [CrossRef]
- Pramod, C.V.; Seshan, K. Catalytic Gasification of Lignocellulosic Biomass; Springer: Singapore, 2016; pp. 173–198. [Google Scholar] [CrossRef]
- Schmid, J.C.; Benedikt, F.; Fuchs, J.; Mauerhofer, A.M.; Müller, S.; Hofbauer, H. Syngas for Biorefineries from Thermochemical Gasification of Lignocellulosic Fuels and Residues—5 Years’ Experience with an Advanced Dual Fluidized Bed Gasifier Design. Biomass Convers. Biorefin. 2019, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Saradha Devi, G.; Vaishnavi, S.; Srinath, S.; Dutt, B.; Rajmohan, K.S. Energy Recovery from Biomass Using Gasification. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 363–382. [Google Scholar] [CrossRef]
- Zhang, K.; Chang, J.; Guan, Y.; Chen, H.; Yang, Y.; Jiang, J. Lignocellulosic Biomass Gasification Technology in China. Renew. Energy 2013, 49, 175–184. [Google Scholar] [CrossRef]
- Jeon, S.J.; Jeong, S.H.; Kim, B.J.; Lee, U.D. Gasification Technologies for Lignocellulosic Biomass. In Emerging Areas in Bioengineering; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; pp. 215–254. [Google Scholar] [CrossRef]
- Alauddin, Z.A.B.Z.; Lahijani, P.; Mohammadi, M.; Mohamed, A.R. Gasification of Lignocellulosic Biomass in Fluidized Beds for Renewable Energy Development: A Review. Renew. Sustain. Energy Rev. 2010, 14, 2852–2862. [Google Scholar] [CrossRef]
- Ibarra-Gonzalez, P.; Rong, B.G. A Review of the Current State of Biofuels Production from Lignocellulosic Biomass Using Thermochemical Conversion Routes. Chin. J. Chem. Eng. 2019, 27, 1523–1535. [Google Scholar] [CrossRef]
- Maitlis, P.; de Klerk, A. Greener Fischer-Tropsch Processes: For Fuels and Feedstocks; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Krishna, B.B.; Biswas, B.; Bhaskar, T. Gasification of Lignocellulosic Biomass. In Biomass, Biofuels, Biochemicals: Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 285–300. [Google Scholar] [CrossRef]
- Lu, Q.; Li, W.Z.; Zhu, X.F. Overview of Fuel Properties of Biomass Fast Pyrolysis Oils. Energy Convers. Manag. 2009, 50, 1376–1383. [Google Scholar] [CrossRef]
- Elliott, D.C. Transportation Fuels from Biomass via Fast Pyrolysis and Hydroprocessing. Wiley Interdiscip. Rev. Energy Environ. 2013, 2, 525–533. [Google Scholar] [CrossRef]
- Verardi, A.; Lopresto, C.G.; Blasi, A.; Chakraborty, S.; Calabrò, V. Bioconversion of Lignocellulosic Biomass to Bioethanol and Biobutanol. In Lignocellulosic Biomass to Liquid Biofuels; Elsevier: Amsterdam, The Netherlands, 2020; pp. 67–125. [Google Scholar] [CrossRef]
- Taha, M.; Foda, M.; Shahsavari, E.; Aburto-Medina, A.; Adetutu, E.; Ball, A. Commercial Feasibility of Lignocellulose Biodegradation: Possibilities and Challenges. Curr. Opin. Biotechnol. 2016, 38, 190–197. [Google Scholar] [CrossRef]
- Althuri, A.; Chintagunta, A.D.; Sherpa, K.C.; Banerjee, R. Simultaneous Saccharification and Fermentation of Lignocellulosic Biomass; Springer: Cham, Switzerland, 2018; pp. 265–285. [Google Scholar] [CrossRef]
- Valdez-Vazquez, I.; Pérez-Rangel, M.; Tapia, A.; Buitrón, G.; Molina, C.; Hernández, G.; Amaya-Delgado, L. Hydrogen and Butanol Production from Native Wheat Straw by Synthetic Microbial Consortia Integrated by Species of Enterococcus and Clostridium. Fuel 2015, 159, 214–222. [Google Scholar] [CrossRef]
- Chen, C.C.; Chuang, Y.S.; Lin, C.Y.; Lay, C.H.; Sen, B. Thermophilic Dark Fermentation of Untreated Rice Straw Using Mixed Cultures for Hydrogen Production. Int. J. Hydrog. Energy 2012, 37, 15540–15546. [Google Scholar] [CrossRef]
- Saggi, S.K.; Dey, P. An Overview of Simultaneous Saccharification and Fermentation of Starchy and Lignocellulosic Biomass for Bio-Ethanol Production. Biofuels 2019, 10, 287–299. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Hasunuma, T.; Ogino, C.; Kondo, A. Bioprocessing of Bio-Based Chemicals Produced from Lignocellulosic Feedstocks. Curr. Opin. Biotechnol. 2016, 42, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.K.; Sharma, S. Recent Updates on Different Methods of Pretreatment of Lignocellulosic Feedstocks: A Review. Bioresour. Bioprocess. 2017, 4, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, F.R.; Khalid, H.; Zhang, H.; Rahman, S.; Zhang, R.; Liu, G.; Chen, C. Pretreatment Methods of Lignocellulosic Biomass for Anaerobic Digestion. AMB Express 2017, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, A.; Sotenko, M.; Blenkinsopp, T.; Coles, S.R. Life Cycle Assessment of Lignocellulosic Biomass Pretreatment Methods in Biofuel Production. Int. J. Life Cycle Assess 2016, 21. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Rehmann, L. Extrusion Pretreatment of Lignocellulosic Biomass: A Review. Int. J. Mol. Sci. 2014, 15, 18967–18984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duque, A.; Manzanares, P.; Ballesteros, M. Extrusion as a Pretreatment for Lignocellulosic Biomass: Fundamentals and Applications. Renew. Energy 2017, 114, 1427–1441. [Google Scholar] [CrossRef]
- Sankaran, R.; Parra Cruz, R.A.; Pakalapati, H.; Show, P.L.; Ling, T.C.; Chen, W.H.; Tao, Y. Recent Advances in the Pretreatment of Microalgal and Lignocellulosic Biomass: A Comprehensive Review. Bioresour. Technol. 2020, 298, 122476. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.; Zhang, H.; Zhang, Q.; Huang, H. Screw Extrude Steam Explosion: A Promising Pretreatment of Corn Stover to Enhance Enzymatic Hydrolysis. Bioresour. Technol. 2014, 161, 230–235. [Google Scholar] [CrossRef]
- Chen, W.H.; Pen, B.L.; Yu, C.T.; Hwang, W.S. Pretreatment Efficiency and Structural Characterization of Rice Straw by an Integrated Process of Dilute-Acid and Steam Explosion for Bioethanol Production. Bioresour. Technol. 2011, 102, 2916–2924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Keshwani, D.R.; Xu, Y.; Hanna, M.A. Alkali Combined Extrusion Pretreatment of Corn Stover to Enhance Enzyme Saccharification. Ind. Crops Prod. 2012, 37, 352–357. [Google Scholar] [CrossRef]
- US8669064B2—Process for providing ethanol from plant material—Google Patents. Available online: https://patents.google.com/patent/US8669064B2/en?oq=U.S.+Patent+No.+8%2C669%2C064.+ (accessed on 25 February 2021).
- US6176176B1—Apparatus for Treating Cellulosic Materials—Google Patents. Available online: https://patents.google.com/patent/US6176176B1/en (accessed on 21 September 2020).
- Yan, X.; Wu, Z.Z.; Li, M.Y.; Yin, F.; Ren, K.X.; Tao, H. The Combined Effects of Extrusion and Heat-Moisture Treatment on the Physicochemical Properties and Digestibility of Corn Starch. Int. J. Biol. Macromol. 2019, 134, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Anushree; Kumar, J.; Bhaskar, T. Utilization of Lignin: A Sustainable and Eco-Friendly Approach. J. Energy Inst. 2020, 93, 235–271. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of Promising Technologies for Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Mayer-Laigle, C.; Solhy, A.; Arancon, R.A.D.; De Vries, H.; Luque, R. Mechanical Pretreatments of Lignocellulosic Biomass: Towards Facile and Environmentally Sound Technologies for Biofuels Production. RSC Adv. 2014, 48109–48127. [Google Scholar] [CrossRef]
- Jȩdrzejczyk, M.; Soszka, E.; Czapnik, M.; Ruppert, A.M.; Grams, J. Physical and Chemical Pretreatment of Lignocellulosic Biomass. In Second and Third Generation of Feedstocks: The Evolution of Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 143–196. [Google Scholar] [CrossRef]
- Yachmenev, V.; Condon, B.; Klasson, T.; Lambert, A. Acceleration of the Enzymatic Hydrolysis of Corn Stover and Sugar Cane Bagasse Celluloses by Low Intensity Uniform Ultrasound. J. Biobased Mater. Bioenergy 2009, 3, 25–31. [Google Scholar] [CrossRef]
- Bussemaker, M.J.; Zhang, D. Effect of Ultrasound on Lignocellulosic Biomass as a Pretreatment for Biorefinery and Biofuel Applications. Ind. Eng. Chem. Res. 2013, 5, 3563–3580. [Google Scholar] [CrossRef]
- Meneses, D.B.; Montes De Oca-Vásquez, G.; Roberto Vega-Baudrit, J.; Rojas-Álvarez, M.; Corrales-Castillo, J.; Murillo-Araya, L.C. Pretreatment Methods of Lignocellulosic Wastes into Value-Added Products: Recent Advances and Possibilities. Biomass Convers. Biorefin. 2020. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging Technologies for the Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Stevens, M.A.; Zhu, Y.; Holmes, J.; Xu, H. Understanding of Alkaline Pretreatment Parameters for Corn Stover Enzymatic Saccharification. Biotechnol. Biofuels 2013, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A Review on Alkaline Pretreatment Technology for Bioconversion of Lignocellulosic Biomass. Bioresour. Technol 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, J.; Baskar, C. Lignocellulosic Biomass for Bioethanol Production through Microbes: Strategies to Improve Process Efficiency; Springer: Cham, Switzerland, 2019; pp. 357–386. [Google Scholar] [CrossRef]
- Solarte-Toro, J.C.; Romero-García, J.M.; Martínez-Patiño, J.C.; Ruiz-Ramos, E.; Castro-Galiano, E.; Cardona-Alzate, C.A. Acid Pretreatment of Lignocellulosic Biomass for Energy Vectors Production: A Review Focused on Operational Conditions and Techno-Economic Assessment for Bioethanol Production. Renew. Sustain. Energy Rev. 2019, 107, 587–601. [Google Scholar] [CrossRef]
- Gonzales, R.R.; Sivagurunathan, P.; Kim, S.H. Effect of Severity on Dilute Acid Pretreatment of Lignocellulosic Biomass and the Following Hydrogen Fermentation. Int. J. Hydrog. Energy 2016, 41, 21678–21684. [Google Scholar] [CrossRef]
- Rezania, S.; Din, M.F.M.; Taib, S.M.; Mohamad, S.E.; Dahalan, F.A.; Kamyab, H.; Darajeh, N.; Ebrahimi, S.S. Ethanol Production from Water Hyacinth (Eichhornia Crassipes) Using Various Types of Enhancers Based on the Consumable Sugars. Waste Biomass Valorization 2018, 9, 939–946. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Gupta, P.; Karpichev, Y.; Gathergood, N.; Bhat, R.; Gupta, V.K. Ionic Liquid Based Pretreatment of Lignocellulosic Biomass for Enhanced Bioconversion. Bioresour. Technol. 2020, 304, 123003. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ju, X.; Zhou, M.; Xu, X.; Fu, J.; Li, L. An Enhanced Ionic Liquid-Tolerant Immobilized Cellulase System via Hydrogel Microsphere for Improving in Situ Saccharification of Biomass. Bioresour. Technol. 2019, 294, 122146. [Google Scholar] [CrossRef]
- Asim, A.M.; Uroos, M.; Naz, S.; Sultan, M.; Griffin, G.; Muhammad, N.; Khan, A.S. Acidic Ionic Liquids: Promising and Cost-Effective Solvents for Processing of Lignocellulosic Biomass. J. Mol. Liq. 2019, 110943, 287. [Google Scholar] [CrossRef]
- Mahmood, H.; Moniruzzaman, M.; Iqbal, T.; Khan, M.J. Recent Advances in the Pretreatment of Lignocellulosic Biomass for Biofuels and Value-Added Products. Curr. Opin. Green Sustain. Chem. 2019, 20, 18–24. [Google Scholar] [CrossRef]
- Shuai, L.; Luterbacher, J. Organic Solvent Effects in Biomass Conversion Reactions. ChemSusChem 2016, 9, 133–155. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.; Wang, D. Organic Solvent Pretreatment of Lignocellulosic Biomass for Biofuels and Biochemicals: A Review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Singh, R. Pretreatment of Lignocellulosic Wastes for Biofuel Production: A Critical Review. Renew. Sust. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Duque, A.; Manzanares, P.; Ballesteros, I.; Ballesteros, M. Steam Explosion as Lignocellulosic Biomass Pretreatment. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 349–368. [Google Scholar] [CrossRef]
- Donohoe, B.S.; Decker, S.R.; Tucker, M.P.; Himmel, M.E.; Vinzant, T.B. Visualizing Lignin Coalescence and Migration through Maize Cell Walls Following Thermochemical Pretreatment. Biotechnol. Bioeng. 2008, 101, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zhou, N.; Li, H.; Deng, W.; Cheng, Y.; Wang, Y.; Liu, Y.; Cobb, K.; Lei, H.; Chen, P.; et al. Recent Advances in Improving Lignocellulosic Biomass-Based Bio-Oil Production. J. Anal. Appl. Pyrolysis 2020, 149, 104845. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Conrad, M.; Sun, S.N.; Sanchez, A.; Rocha, G.J.M.; Romaní, A.; Castro, E.; Torres, A.; Rodríguez-Jasso, R.M.; Andrade, L.P.; et al. Engineering Aspects of Hydrothermal Pretreatment: From Batch to Continuous Operation, Scale-up and Pilot Reactor under Biorefinery Concept. Bioresour. Technol. 2020, 122685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, T.; Held, M.A.; Faik, A. Supercritical CO2 and Ionic Liquids for the Pretreatment of Lignocellulosic Biomass in Bioethanol Production. Environ. Technol. 2013, 34, 1735–1749. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, A.W.; Qureshi, K.; Harijan, K.; Abro, R.; Abbas, T.; Bazmi, A.A.; Karim, S.; Yu, G. Insight into Progress in Pre-Treatment of Lignocellulosic Biomass. Energy 2017, 122, 724–745. [Google Scholar] [CrossRef]
- Bonner, I.J.; Thompson, D.N.; Plummer, M.; Dee, M.; Tumuluru, J.S.; Pace, D.; Teymouri, F.; Campbell, T.; Bals, B. Impact of Ammonia Fiber Expansion (AFEX) Pretreatment on Energy Consumption during Drying, Grinding, and Pelletization of Corn Stover. Dry. Technol. 2016, 34, 1319–1329. [Google Scholar] [CrossRef]
- Abdul, P.M.; Jahim, J.M.; Harun, S.; Markom, M.; Lutpi, N.A.; Hassan, O.; Balan, V.; Dale, B.E.; Mohd Nor, M.T. Effects of Changes in Chemical and Structural Characteristic of Ammonia Fibre Expansion (AFEX) Pretreated Oil Palm Empty Fruit Bunch Fibre on Enzymatic Saccharification and Fermentability for Biohydrogen. Bioresour. Technol. 2016, 211, 200–208. [Google Scholar] [CrossRef]
- Bajpai, P. Pretreatment of Lignocellulosic Biomass for Biofuel Production. Green Chem. Sustain. 2016, 34, 86. [Google Scholar] [CrossRef]
- Hildén, K.; Mäkelä, M.R. Role of Fungi in Wood Decay. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Liu, F.; Monroe, E.W.; Davis, R. Engineering Microbial Consortia for Bioconversion of Multisubstrate Biomass Streams to Biofuels. In Biofuels—Challenges and opportunities; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Darabzadeh, N.; Hamidi-Esfahani, Z.; Hejazi, P. Optimization of Cellulase Production under Solid-state Fermentation by a New Mutant Strain of Trichoderma Reesei. Food Sci. Nutr. 2019, 7, 572–578. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Rodríguez, N.; García-Bernet, D.; Domínguez, J.M. Faster Methane Production after Sequential Extrusion and Enzymatic Hydrolysis of Vine Trimming Shoots. Environ. Chem. Lett. 2018, 16, 295–299. [Google Scholar] [CrossRef]
- Naresh Kumar, M.; Ravikumar, R.; Thenmozhi, S.; Ranjith Kumar, M.; Kirupa Shankar, M. Choice of Pretreatment Technology for Sustainable Production of Bioethanol from Lignocellulosic Biomass: Bottle Necks and Recommendations. Waste Biomass Valorization 2019, 10, 1693–1709. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.X.; Hou, T.; Chen, X.; Gao, C.; Han, L.; Xiao, W. Mechanical Deconstruction of Corn Stover as an Entry Process to Facilitate the Microwave-Assisted Production of Ethyl Levulinate. Fuel Process. Technol. 2018, 174, 53–60. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, P.; Ye, J.; Wu, Y.; Liu, J.; Fang, W.; Xu, D.; Wang, B.; Yan, L.; Zeng, G. Comparison of Various Pretreatments for Ethanol Production Enhancement from Solid Residue after Rumen Fluid Digestion of Rice Straw. Bioresour. Technol. 2018, 247, 147–156. [Google Scholar] [CrossRef]
- El Achkar, J.H.; Lendormi, T.; Salameh, D.; Louka, N.; Maroun, R.G.; Lanoisellé, J.L.; Hobaika, Z. Influence of Pretreatment Conditions on Lignocellulosic Fractions and Methane Production from Grape Pomace. Bioresour. Technol. 2018, 247, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Hernández, M.G.; Ochoa-Martínez, L.A.; Rutiaga-Quiñones, J.G.; Rocha-Guzmán, N.E.; Lara-Ceniceros, T.E.; Contreras-Esquivel, J.C.; Prado Barragán, L.A.; Rutiaga-Quiñones, O.M. Effect of Ultrasound Pre-Treatment on the Physicochemical Composition of Agave Durangensis Leaves and Potential Enzyme Production. Bioresour. Technol. 2018, 249, 439–446. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Pulsed Electric Field Pretreatment of Switchgrass and Wood Chip Species for Biofuel Production. Ind. Eng. Chem. Res. 2011, 50, 10996–11001. [Google Scholar] [CrossRef]
- Molaverdi, M.; Karimi, K.; Mirmohamadsadeghi, S. Improvement of Dry Simultaneous Saccharification and Fermentation of Rice Straw to High Concentration Ethanol by Sodium Carbonate Pretreatment. Energy 2019, 167, 654–660. [Google Scholar] [CrossRef]
- Abdulkhani, A.; Amiri, E.; Sharifzadeh, A.; Hedjazi, S.; Alizadeh, P. Concurrent Production of Sodium Lignosulfonate and Ethanol from Bagasse Spent Liquor. J. Environ. Manag. 2019, 231, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wen, Y.; Kapu, N.S. Ethanol Production from Bamboo Using Mild Alkaline Pre-Extraction Followed by Alkaline Hydrogen Peroxide Pretreatment. Bioresour. Technol. 2018, 247, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xu, Z.; Liu, L.; Chen, S.; Wang, S.; Jin, M. Process Integration for Ethanol Production from Corn and Corn Stover as Mixed Substrates. Bioresour. Technol. 2019, 279, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Malav, M.K.; Kumar, S.; Singh, A.; Pant, D.; Radhakrishnan, S. Enhancement of Bio-Ethanol Production Potential of Wheat Straw by Reducing Furfural and 5-Hydroxymethylfurfural (HMF). Bioresour. Technol. Rep. 2018, 4, 50–56. [Google Scholar] [CrossRef]
- Nasirpour, N.; Mousavi, S.M. RSM Based Optimization of PEG Assisted Ionic Liquid Pretreatment of Sugarcane Bagasse for Enhanced Bioethanol Production: Effect of Process Parameters. Biomass Bioenergy 2018, 116, 89–98. [Google Scholar] [CrossRef]
- Ramezani, N.; Sain, M. Thermal and Physiochemical Characterization of Lignin Extracted from Wheat Straw by Organosolv Process. J. Polym. Environ. 2018, 26, 3109–3116. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Chen, Z.; Li, Y.; Wang, R.; Luo, X.; Cai, G.; Li, Y.; Yu, Q.; Lu, J. Ozonolysis Pretreatment of Maize Stover: The Interactive Effect of Sample Particle Size and Moisture on Ozonolysis Process. Bioresour. Technol. 2015, 183, 240–247. [Google Scholar] [CrossRef]
- Bonfiglio, F.; Cagno, M.; Rey, F.; Torres, M.; Böthig, S.; Menéndez, P.; Mussatto, S.I. Pretreatment of Switchgrass by Steam Explosion in a Semi-Continuous Pre-Pilot Reactor. Biomass Bioenergy 2019, 121, 41–47. [Google Scholar] [CrossRef]
- Medina, J.D.C.; Woiciechowski, A.; Filho, A.Z.; Nigam, P.S.; Ramos, L.P.; Soccol, C.R. Steam Explosion Pretreatment of Oil Palm Empty Fruit Bunches (EFB) Using Autocatalytic Hydrolysis: A Biorefinery Approach. Bioresour. Technol. 2016, 199, 173–180. [Google Scholar] [CrossRef]
- Mohan, M.; Banerjee, T.; Goud, V.V. Hydrolysis of Bamboo Biomass by Subcritical Water Treatment. Bioresour. Technol. 2015, 191, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-J.; Xu, Q.-Q.; Li, G.-m.; Zhang, Q.-Z.; Zhou, D.; Yin, J.-Z.; Zhan, H.-S. Pretreatment of Agricultural Residues by Supercritical CO2 at 50–80 °C to Enhance Enzymatic Hydrolysis. J. Energy Chem. 2019, 31, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Perez-Pimienta, J.A.; Flores-Gómez, C.A.; Ruiz, H.A.; Sathitsuksanoh, N.; Balan, V.; da Costa Sousa, L.; Dale, B.E.; Singh, S.; Simmons, B.A. Evaluation of Agave Bagasse Recalcitrance Using AFEXTM, Autohydrolysis, and Ionic Liquid Pretreatments. Bioresour. Technol. 2016, 211, 216–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Rezende, C.A.; Simister, R.; McQueen-Mason, S.J.; Macquarrie, D.J.; Polikarpov, I.; Gomez, L.D. Efficient Sugar Production from Sugarcane Bagasse by Microwave Assisted Acid and Alkali Pretreatment. Biomass Bioenergy 2016, 93, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Morone, A.; Chakrabarti, T.; Pandey, R.A. Assessment of Alkaline Peroxide-Assisted Wet Air Oxidation Pretreatment for Rice Straw and Its Effect on Enzymatic Hydrolysis. Cellulose 2017, 24, 4885–4898. [Google Scholar] [CrossRef]
- García-Torreiro, M.; López-Abelairas, M.; Lu-Chau, T.A.; Lema, J.M. Fungal Pretreatment of Agricultural Residues for Bioethanol Production. Ind. Crops Prod. 2016, 89, 486–492. [Google Scholar] [CrossRef]
- Dhiman, S.S.; Haw, J.R.; Kalyani, D.; Kalia, V.C.; Kang, Y.C.; Lee, J.K. Simultaneous Pretreatment and Saccharification: Green Technology for Enhanced Sugar Yields from Biomass Using a Fungal Consortium. Bioresour. Technol. 2015, 179, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Srivastava, M.; Ramteke, P.W.; Mishra, P.K. Synthetic Biology Strategy for Microbial Cellulases. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 229–238. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Luo, L.; Wang, E.; Wang, R.; Liu, L.; Liu, J.; Yuan, H. Low-Cost Cellulase-Hemicellulase Mixture Secreted by Trichoderma harzianum EM0925 with Complete Saccharification Efficacy of Lignocellulose. Int. J. Mol. Sci. 2020, 21, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, F.C.; Silvello, M.A.; Goldbeck, R. Cellulase and Oxidative Enzymes: New Approaches, Challenges and Perspectives on Cellulose Degradation for Bioethanol Production. Biotechnol. Lett. 2020, 42, 875–884. [Google Scholar] [CrossRef]
- Valenzuela, S.V.; Ferreres, G.; Margalef, G.; Pastor, F.I.J. Fast Purification Method of Functional LPMOs from Streptomyces Ambofaciens by Affinity Adsorption. Carbohydr. Res. 2017, 448, 205–211. [Google Scholar] [CrossRef]
- Zhang, R. Functional Characterization of Cellulose-Degrading AA9 Lytic Polysaccharide Monooxygenases and Their Potential Exploitation. Appl. Microbiol. Biotechnol. 2020, 104, 3229–3243. [Google Scholar] [CrossRef]
- Song, B.; Li, B.; Wang, X.; Shen, W.; Park, S.; Collings, C.; Feng, A.; Smith, S.J.; Walton, J.D.; Ding, S.Y. Real-Time Imaging Reveals That Lytic Polysaccharide Monooxygenase Promotes Cellulase Activity by Increasing Cellulose Accessibility. Biotechnol. Biofuels 2018, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Dinamarco, T.M.; Goldbeck, R. Recombinant Chimeric Enzymes for Lignocellulosic Biomass Hydrolysis. Enzyme Microb. Technol. 2020, 109647. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Bibra, M.; Chadha, B.S.; Sani, R.K. Enhanced Hydrolysis of Lignocellulosic Biomass with Doping of a Highly Thermostable Recombinant Laccase. Int. J. Biol. Macromol. 2019, 137, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Brenelli, L.; Squina, F.M.; Felby, C.; Cannella, D. Laccase-Derived Lignin Compounds Boost Cellulose Oxidative Enzymes AA9. Biotechnol. Biofuels 2018, 11, 10. [Google Scholar] [CrossRef]
- Karnaouri, A.; Muraleedharan, M.N.; Dimarogona, M.; Topakas, E.; Rova, U.; Sandgren, M.; Christakopoulos, P. Recombinant Expression of Thermostable Processive MtEG5 Endoglucanase and Its Synergism with MtLPMO from Myceliophthora Thermophila during the Hydrolysis of Lignocellulosic Substrates. Biotechnol. Biofuels 2017, 10, 1–17. [Google Scholar] [CrossRef]
- Katahira, S.; Ito, M.; Takema, H.; Fujita, Y.; Tanino, T.; Tanaka, T.; Fukuda, H.; Kondo, A. Improvement of Ethanol Productivity during Xylose and Glucose Co-Fermentation by Xylose-Assimilating, S. Cerevisiae via Expression of Glucose Transporter Sut1. Enzym. Microb. Technol. 2008, 43, 115–119. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, L. Lignocelluloses Feedstock Biorefinery as Petrorefinery Substitutes. In Biomass Now—Sustainable Growth and Use; InTech: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Tomani, P. The Lignoboost Process. Cellul. Chem. Technol. 2010, 44, 53. [Google Scholar]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Moving towards the Second Generation of Lignocellulosic Biorefineries in the EU: Drivers, Challenges, and Opportunities. Renew. Sust. Energy Rev. 2019, 590–599. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Lignocellulosic Biorefineries in Europe: Current State and Prospects. Trends Biotechnol. 2019, 231–234. [Google Scholar] [CrossRef] [Green Version]
- Arora, A.; Nandal, P.; Singh, J.; Verma, M.L. Nanobiotechnological Advancements in Lignocellulosic Biomass Pretreatment. Mater. Sci. Energy Technol. 2020, 3, 308–318. [Google Scholar] [CrossRef]
- Anukam, A.; Berghel, J. Biomass Pretreatment and Characterization: A Review. In Biomass; IntechOpen: Londn, UK, 2020. [Google Scholar] [CrossRef]
- Current Biomass Research Projects—Celignis Biomass Analysis Laboratory. Available online: https://www.celignis.com/current_projects.php (accessed on 16 February 2021).
- Topic Industry highlights—EUBCE 2021|29th European Biomass Conference and Exhibition. Available online: https://www.eubce.com/topic-industry-highlights.html (accessed on 16 February 2021).
- Saini, R.; Osorio-Gonzalez, C.S.; Hegde, K.; Brar, S.K.; Magdouli, S.; Vezina, P.; Avalos-Ramirez, A. Lignocellulosic Biomass-Based Biorefinery: An Insight into Commercialization and Economic Standout. Curr. Sustain. Energy Rep. 2020, 7, 122–136. [Google Scholar] [CrossRef]
- Rodrigues, M.F.F.; Sousa, I.M.O.; Vardanega, R.; Nogueira, G.C.; Meireles, M.A.A.; Foglio, M.A.; Marchese, J.A. Techno-Economic Evaluation of Artemisinin Extraction from Artemisia Annua L. Using Supercritical Carbon Dioxide. Ind. Crops Prod. 2019, 132, 336–343. [Google Scholar] [CrossRef]
- Rodrigues Gurgel da Silva, A.; Giuliano, A.; Errico, M.; Rong, B.G.; Barletta, D. Economic Value and Environmental Impact Analysis of Lignocellulosic Ethanol Production: Assessment of Different Pretreatment Processes. Clean Technol. Environ. Policy 2019, 21, 637–654. [Google Scholar] [CrossRef]
- Maity, S.K. Opportunities, Recent Trends and Challenges of Integrated Biorefinery: Part I. Renew. Sust. Energy Rev. 2015, 1427–1445. [Google Scholar] [CrossRef] [Green Version]
- Paone, E.; Tabanelli, T.; Mauriello, F. The Rise of Lignin Biorefinery. Curr. Opin. Green Sustain. Chem. 2020, 1–6. [Google Scholar] [CrossRef]
- Maity, S.K. Opportunities, Recent Trends and Challenges of Integrated Biorefinery: Part II. Renew. Sust. Energy Rev. 2015, 1446–1466. [Google Scholar] [CrossRef] [Green Version]
- Sutton, D.; Kelleher, B.; Ross, J.R.H. Review of Literature on Catalysts for Biomass Gasification. Fuel Process. Technol. 2001, 155–173. [Google Scholar] [CrossRef]
- Maghanki, M.M.; Ghobadian, B.; Najafi, G.; Galogah, R.J. Micro Combined Heat and Power (MCHP) Technologies and Applications. Renew. Sust. Energy Rev. 2013, 510–524. [Google Scholar] [CrossRef]
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Antunes, F.A.F.; da Silva, S.S. The Path Forward for Lignocellulose Biorefineries: Bottlenecks, Solutions, and Perspective on Commercialization. Bioresour. Technol. 2018, 370–381. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haq, I.u.; Qaisar, K.; Nawaz, A.; Akram, F.; Mukhtar, H.; Zohu, X.; Xu, Y.; Mumtaz, M.W.; Rashid, U.; Ghani, W.A.W.A.K.; et al. Advances in Valorization of Lignocellulosic Biomass towards Energy Generation. Catalysts 2021, 11, 309. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11030309
Haq Iu, Qaisar K, Nawaz A, Akram F, Mukhtar H, Zohu X, Xu Y, Mumtaz MW, Rashid U, Ghani WAWAK, et al. Advances in Valorization of Lignocellulosic Biomass towards Energy Generation. Catalysts. 2021; 11(3):309. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11030309
Chicago/Turabian StyleHaq, Ikram ul, Kinza Qaisar, Ali Nawaz, Fatima Akram, Hamid Mukhtar, Xin Zohu, Yong Xu, Muhammad Waseem Mumtaz, Umer Rashid, Wan Azlina Wan Ab Karim Ghani, and et al. 2021. "Advances in Valorization of Lignocellulosic Biomass towards Energy Generation" Catalysts 11, no. 3: 309. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11030309