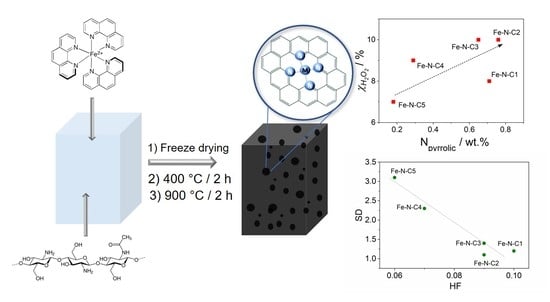
Highly Graphitized Fe-N-C Electrocatalysts Prepared from Chitosan Hydrogel Frameworks
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological and Textural Properties
2.2. Chemical and Spectroscopic Characterization
2.3. ORR Electrochemical Performace
3. Materials and Methods
3.1. Synthesis of the Catalysts
3.2. Electrochemical Test
3.3. Physico-Chemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.; Shen, X.; Pan, Y.; Peng, Z. A review of Pt-based electrocatalysts for oxygen reduction reaction. Front. Energy 2017, 11, 268–285. [Google Scholar] [CrossRef]
- Stephens, I.E.L.; Bondarenko, A.S.; Grønbjerg, U.; Rossmeisl, J.; Chorkendorff, I. Understanding the electrocatalysis of oxygen reduction on platinum and its alloys. Energy Environ. Sci. 2012, 5, 6744–6762. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Shen, X.; Pan, Y.; Yao, L.; Peng, Z. Platinum Alloy Catalysts for Oxygen Reduction Reaction: Advances, Challenges and Perspectives. ChemNanoMat 2020, 6, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Zamburlini, E.; Jensen, K.D.; Stephens, I.E.L.; Chorkendorff, I.; Escudero-Escribano, M. Benchmarking Pt and Pt-lanthanide sputtered thin films for oxygen electroreduction: Fabrication and rotating disk electrode measurements. Electrochim. Acta 2017, 247, 708–721. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.; Vorokhta, M.; Khalakhan, I.; Dopita, M.; Vonderach, T.; Skála, T.; Lindahl, N.; Matolínová, I.; Grönbeck, H.; Neyman, K.M.; et al. Unraveling the Surface Chemistry and Structure in Highly Active Sputtered Pt3Y Catalyst Films for the Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2020, 12, 4454–4462. [Google Scholar] [CrossRef] [PubMed]
- Brandiele, R.; Guadagnini, A.; Girardi, L.; Dražić, G.; Dalconi, M.C.; Rizzi, G.A.; Amendola, V.; Durante, C. Climbing the oxygen reduction reaction volcano plot with laser ablation synthesis of PtxY nanoalloys. Catal. Sci. Technol. 2020, 10, 4503–4508. [Google Scholar] [CrossRef]
- Brandiele, R.; Durante, C.; Grądzka, E.; Rizzi, G.A.; Zheng, J.; Badocco, D.; Centomo, P.; Pastore, P.; Granozzi, G.; Gennaro, A. One step forward to a scalable synthesis of platinum–yttrium alloy nanoparticles on mesoporous carbon for the oxygen reduction reaction. J. Mater. Chem. A 2016, 4, 12232–12240. [Google Scholar] [CrossRef]
- Meier, J.C.; Galeano, C.; Katsounaros, I.; Witte, J.; Bongard, H.J.; Topalov, A.A.; Baldizzone, C.; Mezzavilla, S.; Schüth, F.; Mayrhofer, K.J.J. Design criteria for stable Pt/C fuel cell catalysts. Beilstein J. Nanotechnol. 2014, 5, 44–67. [Google Scholar] [CrossRef] [Green Version]
- Vidal-Iglesias, F.J.; Arán-Ais, R.M.; Solla-Gullón, J.; Herrero, E.; Feliu, J.M. Electrochemical Characterization of Shape-Controlled Pt Nanoparticles in Different Supporting Electrolytes. ACS Catal. 2012, 2, 901–910. [Google Scholar] [CrossRef] [Green Version]
- Brandiele, R.; Zerbetto, M.; Dalconi, M.C.; Rizzi, G.A.; Isse, A.A.; Durante, C.; Gennaro, A. Mesoporous Carbon with Different Density of Thiophenic-Like Functional Groups and Their Effect on Oxygen Reduction. ChemSusChem 2019, 12, 4229–4239. [Google Scholar] [CrossRef]
- Brandiele, R.; Durante, C.; Zerbetto, M.; Vicentini, N.; Kosmala, T.; Badocco, D.; Pastore, P.; Rizzi, G.A.; Isse, A.A.; Gennaro, A. Probing the correlation between Pt-support interaction and oxygen reduction reaction activity in mesoporous carbon materials modified with Pt-N active sites. Electrochim. Acta 2018, 277, 287–300. [Google Scholar] [CrossRef]
- Perazzolo, V.; Brandiele, R.; Durante, C.; Zerbetto, M.; Causin, V.; Rizzi, G.A.; Cerri, I.; Granozzi, G.; Gennaro, A. Density Functional Theory (DFT) and Experimental Evidences of Metal–Support Interaction in Platinum Nanoparticles Supported on Nitrogen- and Sulfur-Doped Mesoporous Carbons: Synthesis, Activity, and Stability. ACS Catal. 2018, 8, 1122–1137. [Google Scholar] [CrossRef]
- Primbs, M.; Sun, Y.; Roy, A.; Malko, D.; Mehmood, A.; Sougrati, M.-T.; Blanchard, P.-Y.; Granozzi, G.; Kosmala, T.; Daniel, G.; et al. Establishing Reactivity Descriptors for Platinum Group Metal (PGM)-free Fe-N-C Catalysts for PEM Fuel Cells. Energy Environ. Sci. 2020, 10, 147–154. [Google Scholar] [CrossRef]
- Jaouen, F.; Herranz, J.; Lefèvre, M.; Dodelet, J.-P.; Kramm, U.I.; Herrmann, I.; Bogdanoff, P.; Maruyama, J.; Nagaoka, T.; Garsuch, A.; et al. Cross-Laboratory Experimental Study of Non-Noble-Metal Electrocatalysts for the Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2009, 1, 1623–1639. [Google Scholar] [CrossRef]
- Mineva, T.; Matanovic, I.; Atanassov, P.; Sougrati, M.-T.; Stievano, L.; Clémancey, M.; Kochem, A.; Latour, J.-M.; Jaouen, F. Understanding Active Sites in Pyrolyzed Fe–N–C Catalysts for Fuel Cell Cathodes by Bridging Density Functional Theory Calculations and 57 Fe Mössbauer Spectroscopy. ACS Catal. 2019, 9, 9359–9371. [Google Scholar] [CrossRef]
- Shao, Y.; Dodelet, J.; Wu, G.; Zelenay, P. PGM-Free Cathode Catalysts for PEM Fuel Cells: A Mini-Review on Stability Challenges. Adv. Mater. 2019, 31, 1807615. [Google Scholar] [CrossRef] [PubMed]
- Martinez, U.; Komini Babu, S.; Holby, E.F.; Zelenay, P. Durability challenges and perspective in the development of PGM-free electrocatalysts for the oxygen reduction reaction. Curr. Opin. Electrochem. 2018, 9, 224–232. [Google Scholar] [CrossRef]
- Martinaiou, I.; Shahraei, A.; Grimm, F.; Zhang, H.; Wittich, C.; Klemenz, S.; Dolique, S.J.; Kleebe, H.-J.; Stark, R.W.; Kramm, U.I. Effect of metal species on the stability of Me-N-C catalysts during accelerated stress tests mimicking the start-up and shut-down conditions. Electrochim. Acta 2017, 243, 183–196. [Google Scholar] [CrossRef]
- Mazzucato, M.; Giorgia, D.; Tomasz, K.; Mahmood, A.; Gaetano, G.; Anthony, K.; Durante, C. Effects of the induced Micro- and Meso-porosity on the single site density and turn over frequency of Fe-N-C carbon electrodes for the Oxygen Reduction Reaction. Appl. Catal. B Environ. 2021, 291, 120068. [Google Scholar] [CrossRef]
- Lai, Y.; Wang, Q.; Wang, M.; Li, J.; Fang, J.; Zhang, Z. Facile synthesis of mesoporous Fe-N-C electrocatalyst for high performance alkaline aluminum-air battery. J. Electroanal. Chem. 2017, 801, 72–76. [Google Scholar] [CrossRef]
- Zhang, X.; Han, X.; Jiang, Z.; Xu, J.; Chen, L.; Xue, Y.; Nie, A.; Xie, Z.; Kuang, Q.; Zheng, L. Atomically dispersed hierarchically ordered porous Fe–N–C electrocatalyst for high performance electrocatalytic oxygen reduction in Zn-Air battery. Nano Energy 2020, 71, 104547–104556. [Google Scholar] [CrossRef]
- Meng, Z.; Stolz, R.M.; Mendecki, L.; Mirica, K.A. Electrically-Transduced Chemical Sensors Based on Two-Dimensional Nanomaterials. Chem. Rev. 2019, 119, 478–598. [Google Scholar] [CrossRef]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Hu, J.; Yang, J.; Xiao, K.; Liang, S.; Xu, J.; Liu, B.; Hou, H. Fe and N co-doped carbon derived from melamine resin capsuled biomass as efficient oxygen reduction catalyst for air-cathode microbial fuel cells. Int. J. Hydrog. Energy 2020, 45, 3163–3175. [Google Scholar] [CrossRef]
- Daniel, G.; Foltran, E.; Brandiele, R.; Nodari, L.; Pilot, R.; Menna, E.; Rizzi, G.A.; Isse, A.A.; Durante, C.; Gennaro, A. Platinum-free electrocatalysts for oxygen reduction reaction: Fe-Nx modified mesoporous carbon prepared from biosources. J. Power Sources 2018, 402, 434–446. [Google Scholar] [CrossRef]
- Artyushkova, K.; Serov, A.; Rojas-Carbonell, S.; Atanassov, P. Chemistry of Multitudinous Active Sites for Oxygen Reduction Reaction in Transition Metal–Nitrogen–Carbon Electrocatalysts. J. Phys. Chem. C 2015, 119, 25917–25928. [Google Scholar] [CrossRef]
- Matanovic, I.; Artyushkova, K.; Atanassov, P. Understanding PGM-free catalysts by linking density functional theory calculations and structural analysis: Perspectives and challenges. Curr. Opin. Electrochem. 2018, 9, 137–144. [Google Scholar] [CrossRef]
- Matanovic, I.; Artyushkova, K.; Strand, M.B.; Dzara, M.J.; Pylypenko, S.; Atanassov, P. Core Level Shifts of Hydrogenated Pyridinic and Pyrrolic Nitrogen in the Nitrogen-Containing Graphene-Based Electrocatalysts: In-Plane vs Edge Defects. J. Phys. Chem. C 2016, 120, 29225–29232. [Google Scholar] [CrossRef]
- Li, J.; Sougrati, M.T.; Zitolo, A.; Ablett, J.M.; Oğuz, I.C.; Mineva, T.; Matanovic, I.; Atanassov, P.; Huang, Y.; Zenyuk, I.; et al. Identification of durable and non-durable FeNx sites in Fe–N–C materials for proton exchange membrane fuel cells. Nat. Catal. 2021, 4, 10–19. [Google Scholar] [CrossRef]
- Yang, W.; Wang, X.; Rossi, R.; Logan, B.E. Low-cost Fe–N–C catalyst derived from Fe (III)-chitosan hydrogel to enhance power production in microbial fuel cells. Chem. Eng. J. 2020, 380, 122522. [Google Scholar] [CrossRef]
- Chenitz, R.; Kramm, U.I.; Lefèvre, M.; Glibin, V.; Zhang, G.; Sun, S.; Dodelet, J.-P. A specific demetalation of Fe–N4 catalytic sites in the micropores of NC_Ar + NH3 is at the origin of the initial activity loss of the highly active Fe/N/C catalyst used for the reduction of oxygen in PEM fuel cells. Energy Environ. Sci. 2018, 11, 365–382. [Google Scholar] [CrossRef]
- Jaouen, F.; Lefèvre, M.; Dodelet, J.-P.; Cai, M. Heat-Treated Fe/N/C Catalysts for O2 Electroreduction: Are Active Sites Hosted in Micropores? J. Phys. Chem. B 2006, 110, 5553–5558. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Li, Z.; Dai, S. Mesoporous Carbon Materials: Synthesis and Modification. Angew. Chem. Int. Ed. 2008, 47, 3696–3717. [Google Scholar] [CrossRef] [PubMed]
- Asset, T.; Atanassov, P. Iron-Nitrogen-Carbon Catalysts for Proton Exchange Membrane Fuel Cells. Joule 2020, 4, 33–44. [Google Scholar] [CrossRef]
- Perazzolo, V.; Durante, C.; Pilot, R.; Paduano, A.; Zheng, J.; Rizzi, G.A.; Martucci, A.; Granozzi, G.; Gennaro, A. Nitrogen and sulfur doped mesoporous carbon as metal-free electrocatalysts for the in situ production of hydrogen peroxide. Carbon 2015, 95, 949–963. [Google Scholar] [CrossRef]
- Perazzolo, V.; Durante, C.; Gennaro, A. Nitrogen and sulfur doped mesoporous carbon cathodes for water treatment. J. Electroanal. Chem. 2016, 782, 264–269. [Google Scholar] [CrossRef]
- Perazzolo, V.; Grądzka, E.; Durante, C.; Pilot, R.; Vicentini, N.; Rizzi, G.A.; Granozzi, G.; Gennaro, A. Chemical and electrochemical stability of nitrogen and sulphur doped mesoporous carbons. Electrochim. Acta 2016, 197, 251–262. [Google Scholar] [CrossRef]
- Brandiele, R.; Picelli, L.; Pilot, R.; Causin, V.; Martucci, A.; Rizzi, G.A.; Isse, A.A.; Durante, C.; Gennaro, A. Nitrogen and Sulfur Doped Mesoporous Carbons, Prepared from Templating Silica, as Interesting Material for Supercapacitors. ChemistrySelect 2017, 2, 7082–7090. [Google Scholar] [CrossRef]
- Brandiele, R.; Poli, F.; Picelli, L.; Pilot, R.; Rizzi, G.A.; Soavi, F.; Durante, C. Nitrogen doped mesoporous carbon electrodes prepared from templating propylamine functionalized silica. ChemElectroChem 2020, 92, 455–459. [Google Scholar] [CrossRef]
- Perazzolo, V.; Daniel, G.; Brandiele, R.; Picelli, L.; Rizzi, G.A.; Isse, A.A.; Durante, C. PEO-b-PS Block Copolymer Templated Mesoporous Carbons: A Comparative Study of Nitrogen and Sulfur Doping in the Oxygen Reduction Reaction to Hydrogen Peroxide. Chem. A Eur. J. 2021, 27, 1002–1014. [Google Scholar] [CrossRef]
- Trevisanello, E.; De Bon, F.; Daniel, G.; Lorandi, F.; Durante, C.; Isse, A.A.; Gennaro, A. Electrochemically mediated atom transfer radical polymerization of acrylonitrile and poly(acrylonitrile-b-butyl acrylate) copolymer as a precursor for N-doped mesoporous carbons. Electrochim. Acta 2018, 285, 344–354. [Google Scholar] [CrossRef]
- Daniel, G.; Kosmala, T.; Dalconi, M.C.; Nodari, L.; Badocco, D.; Pastore, P.; Lorenzetti, A.; Granozzi, G.; Durante, C. Upcycling of polyurethane into iron-nitrogen-carbon electrocatalysts active for oxygen reduction reaction. Electrochim. Acta 2020, 362, 137200. [Google Scholar] [CrossRef]
- Huang, R.; Lei, Y.; Zhang, D.; Xie, H.; Liu, X.; Wang, H. Solvent-Free Assembled Fe-Chitosan Chelates Derived N-Doped Carbon Layer-Encapsulated Fe/Fe 3 C for ORR and OER. Nano 2020, 15, 2050070. [Google Scholar] [CrossRef]
- Wang, W.; Liu, S.; Liu, Y.; Jing, W.; Zhao, R.; Lei, Z. Phenolic resin/chitosan composite derived nitrogen-doped carbon as highly durable and anti-poisoning electrocatalyst for oxygen reduction reaction. Int. J. Hydrog. Energy 2017, 42, 26704–26712. [Google Scholar] [CrossRef]
- Aghabarari, B.; Martínez-Huerta, M.V.; Capel-Sánchez, M.C.; Lázaro, M.J. Non-precious Melamine/Chitosan Composites for the Oxygen Reduction Reaction: Effect of the Transition Metal. Front. Mater. 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Khan, A.; Goepel, M.; Colmenares, J.C.; Gläser, R. Chitosan-Based N-Doped Carbon Materials for Electrocatalytic and Photocatalytic Applications. ACS Sustain. Chem. Eng. 2020, 8, 4708–4727. [Google Scholar] [CrossRef] [Green Version]
- Borghei, M.; Lehtonen, J.; Liu, L.; Rojas, O.J. Advanced Biomass-Derived Electrocatalysts for the Oxygen Reduction Reaction. Adv. Mater. 2018, 30, 1703691. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, C.; Liu, D.; Liu, Y.; Yu, X.; Wang, L. Boosting ORR Electrocatalytic Performance of Metal-Free Mesoporous Biomass Carbon by Synergism of Huge Specific Surface Area and Ultrahigh Pyridinic Nitrogen Doping. ACS Sustain. Chem. Eng. 2018, 6, 13807–13812. [Google Scholar] [CrossRef]
- Daniel, G.; Zhang, Y.; Lanzalaco, S.; Brombin, F.; Kosmala, T.; Granozzi, G.; Wang, A.; Brillas, E.; Sirés, I.; Durante, C. Chitosan-Derived Nitrogen-Doped Carbon Electrocatalyst for a Sustainable Upgrade of Oxygen Reduction to Hydrogen Peroxide in UV-Assisted Electro-Fenton Water Treatment. ACS Sustain. Chem. Eng. 2020, 8, 14425–14440. [Google Scholar] [CrossRef]
- Zhang, G.; Li, L.; Chen, M.; Yang, F. Chitosan cross-linked poly(aminoanthraquinone)/Prussian blue ternary nitrogen precursor-derived Fe–N–C oxygen reduction catalysts for microbial fuel cells and zinc–air batteries. J. Mater. Chem. A 2020, 8, 9256–9267. [Google Scholar] [CrossRef]
- Schmitz, C.; Auza, L.G.; Koberidze, D.; Rasche, S.; Fischer, R.; Bortesi, L. Conversion of chitin to defined chitosan oligomers: Current status and future prospects. Mar. Drugs 2019, 17, 452. [Google Scholar] [CrossRef] [Green Version]
- Zhong, H.; Duan, L.; Ye, P.; Li, X.; Xu, A.; Peng, Q. Synthesis of cobalt–nitrogen-doped mesoporous carbon from chitosan and its performance for pollutant degradation as Fenton-like catalysts. Res. Chem. Intermediat. 2019, 45, 907–918. [Google Scholar] [CrossRef]
- Nie, J.; Wang, Z.; Hu, Q. Difference between Chitosan Hydrogels via Alkaline and Acidic Solvent Systems. Sci. Rep. 2016, 6, 36053. [Google Scholar] [CrossRef] [Green Version]
- Tong, X.; Chen, Z.; Zhuo, H.; Hu, Y.; Jing, S.; Liu, J.; Zhong, L. Tailoring the physicochemical properties of chitosan-derived N-doped carbon by controlling hydrothermal carbonization time for high-performance supercapacitor application. Carbohydr. Polym. 2019, 207, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Jurca, B.; Bucur, C.; Primo, A.; Concepción, P.; Parvulescu, V.I.; García, H. N-Doped Defective Graphene from Biomass as Catalyst for CO2 Hydrogenation to Methane. ChemCatChem 2019, 11, 985–990. [Google Scholar] [CrossRef]
- Liao, C.; Liu, B.; Chi, Q.; Zhang, Z. Nitrogen-Doped Carbon Materials for the Metal-Free Reduction of Nitro Compounds. ACS Appl. Mater. Interfaces 2018, 10, 44421–44429. [Google Scholar] [CrossRef]
- Conder, J.; Vaulot, C.; Marino, C.; Villevieille, C.; Ghimbeu, C. Chitin and chitosan—Structurally-related precursors of dissimilar hard carbons for Na-ion battery. ACS Appl. Energy Mater. 2019, 2, 4841–4852. [Google Scholar] [CrossRef]
- Qiao, Y.; Kong, F.; Zhang, C.; Li, R.; Kong, A.; Shan, Y. Highly efficient oxygen electrode catalyst derived from chitosan biomass by molten salt pyrolysis for zinc-air battery. Electrochim. Acta 2020, 339. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Yang, W.; Zhu, Z.; Zhai, Y.; Gu, W.; Zhu, C. Multiscale porous Fe-N-C networks as highly efficient catalysts for the oxygen reduction reaction. Nanoscale 2019, 11, 19506–19511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, L.; Zhang, S.; Lv, Z.; Yang, D.; Liu, J.; Chen, Y.; Tian, X.; Jin, H.; Song, W. Biomass chitosan derived cobalt/nitrogen doped carbon nanotubes for the electrocatalytic oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 5740–5745. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Zhou, Y.; Jia, D.; Song, Y. CS-Fe(II,III) complex as precursor for magnetite nanocrystal. Polym. Adv. Technol. 2011, 22, 1681–1684. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Poddubnaya, A.M.P.O.I.; Sobiesiak, B.G.M. Comparison of heterogeneous pore models QSDFT and 2D-NLDFT and computer programs ASiQwin and SAIEUS for calculation of pore size distribution. Adsorption 2016, 459–464. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Verboekend, D.; Bonilla, A.; Abelló, S. Zeolite Catalysts with Tunable Hierarchy Factor by Pore-Growth Moderators. Adv. Funct. Mater. 2009, 19, 3972–3979. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Pawlyta, M.; Rouzaud, J.-N.; Duber, S. Raman microspectroscopy characterization of carbon blacks: Spectral analysis and structural information. Carbon 2015, 84, 479–490. [Google Scholar] [CrossRef]
- Beyssac, O.; Goffé, B.; Petitet, J.-P.; Froigneux, E.; Moreau, M.; Rouzaud, J.-N. On the characterization of disordered and heterogeneous carbonaceous materials by Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2267–2276. [Google Scholar] [CrossRef]
- Malko, D.; Kucernak, A.; Lopes, T. Performance of Fe–N/C Oxygen Reduction Electrocatalysts toward NO2−, NO, and NH2OH Electroreduction: From Fundamental Insights into the Active Center to a New Method for Environmental Nitrite Destruction. J. Am. Chem. Soc. 2016, 138, 16056–16068. [Google Scholar] [CrossRef] [Green Version]
- Malko, D.; Kucernak, A.; Lopes, T. In situ electrochemical quantification of active sites in Fe–N/C non-precious metal catalysts. Nat. Commun. 2016, 7, 13285–13292. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Xu, L.; Liu, L.; Wang, L.; Zhang, X.; Kong, A. Bio-inspired chitosan-heme-vitamin B 12 -derived Fe–Co bimetallic-doped mesoporous carbons for efficiently electro-activating oxygen. Dalt. Trans. 2019, 48, 2338–2344. [Google Scholar] [CrossRef] [PubMed]






| Samples | Precursors | Details | ||||
|---|---|---|---|---|---|---|
| Chitosan | Phen | Fe(II)Cl2 | Fe(III)Cl3 | Fe(Phen)3Cl2 | Added after First Pyrolysis at 400 °C | |
| Fe-N-C1 | ✔ | ✔ | ✔ | |||
| Fe-N-C2 | ✔ | ✔ | ✔ | |||
| Fe-N-C3 | ✔ | ✔ | ||||
| Fe-N-C4 | ✔ | ✔ | ✔ | |||
| Fe-N-C5 | ✔ | ✔ | ✔ | |||
| Supports | N2 Ads/Desorption Isotherm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C a | N a | Fe b | SQSDFT | VTOT | VQSDFT | Sμ | Sm | HF | ||
| % | % | % | m2 g−1 | cm3 g−1 | cm3 g−1 | m2 g−1 | m2 g−1 | |||
| Fe-N-C1 | 86.78 | 0.44 | n.a. | n.a. | 627 | 0.477 | 0.425 | 396 | 232 | 0.10 |
| Fe-N-C2 | 85.57 | 0.56 | n.a. | n.a. | 255 | 0.214 | 0.182 | 157 | 98 | 0.09 |
| Fe-N-C3 | 81.71 | 0.68 | 15.2 c | 4.9 d | 356 | 0.260 | 0.224 | 248 | 108 | 0.09 |
| Fe-N-C4 | 85.71 | 0.90 | 10.0 c | 0.6 d | 360 | 0.275 | 0.233 | 268 | 91 | 0.07 |
| Fe-N-C5 | 89.25 | 0.45 | 9.6 c | 0.4 d | 233 | 0.337 | 0.253 | 122 | 111 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniel, G.; Kosmala, T.; Brombin, F.; Mazzucato, M.; Facchin, A.; Dalconi, M.C.; Badocco, D.; Pastore, P.; Granozzi, G.; Durante, C. Highly Graphitized Fe-N-C Electrocatalysts Prepared from Chitosan Hydrogel Frameworks. Catalysts 2021, 11, 390. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11030390
Daniel G, Kosmala T, Brombin F, Mazzucato M, Facchin A, Dalconi MC, Badocco D, Pastore P, Granozzi G, Durante C. Highly Graphitized Fe-N-C Electrocatalysts Prepared from Chitosan Hydrogel Frameworks. Catalysts. 2021; 11(3):390. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11030390
Chicago/Turabian StyleDaniel, Giorgia, Tomasz Kosmala, Federico Brombin, Marco Mazzucato, Alessandro Facchin, Maria Chiara Dalconi, Denis Badocco, Paolo Pastore, Gaetano Granozzi, and Christian Durante. 2021. "Highly Graphitized Fe-N-C Electrocatalysts Prepared from Chitosan Hydrogel Frameworks" Catalysts 11, no. 3: 390. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11030390