Influence of Nutrient-Stress Conditions on Chlorella vulgaris Biomass Production and Lipid Content
Abstract
:1. Introduction
2. Results and Discussion
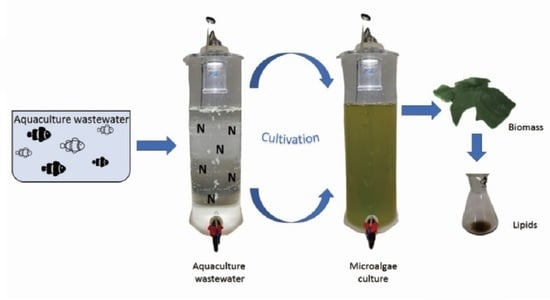
2.1. Production of Chlorella vulgaris Biomass in Aquaculture Wastewater
2.2. Effect of Nitrogen Content on the Biomass and Lipid Content
2.2.1. Production of Biomass under Nutrient-Stress Condition
2.2.2. Optical Density of Microalgal Cultures
2.2.3. Lipid Accumulation under Nitrogen Limitation
2.2.4. Changes in PH during Microalga Cultivation
2.2.5. Chlorophyll A Content in Microalgal Biomass
3. Materials and Methods
3.1. Microalgal Culture
3.2. Aquaculture Wastewater
3.3. Experimental Setup
3.4. Analytical Methods
3.4.1. Biomass Quantification
3.4.2. Pigment Extraction and Analysis
3.4.3. Determination of PH
3.4.4. Lipids Extraction and Determination
3.4.5. Determination of Total Nitrogen Content
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z. Comparisons of various absorbent effects on carbon dioxide capture in membrane gas absorption (MGA) process. J. Nat. Gas Sci. Eng. 2016, 31, 589–595. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Arumugam, M.; Agarwal, A.; Arya, M.C.; Ahmed, Z. Influence of nitrogen sources on biomass productivity of microalgae Scenedesmus bijugatus. Bioresour. Technol. 2013, 131, 246–249. [Google Scholar] [CrossRef]
- John, R.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Micro and macroalgal biomass: A renewable source for bioethanol. Bioresour. Technol. 2011, 102, 186–193. [Google Scholar] [CrossRef]
- Ho, S.H.; Ye, X.; Hasunuma, T.; Chang, J.S.; Kondo, A. Perspectives on engineering strategies for improving biofuels production from microalgae–A critical review. Biotechnol. Adv. 2014, 32, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.P.; Huang, Y.; Liao, Q.; Fu, Q.; Xia, A. Effect of wettability on the growth of Scenedesmus obliquus biofilm attached on glass surface coated with polytetrafluoroethylene emulsion. Int. J. Hydrog. Energy 2016, 41, 21728–21735. [Google Scholar] [CrossRef]
- Babu, A.; Wu, X.; Kabra, A.N.; Kim, D.P. Cultivation an indigenous Chlorella sorokiniana with phytohormones for biomass and lipid production under N-limitations. Algal Res. 2017, 23, 178–185. [Google Scholar] [CrossRef]
- Singh, P.; Kumari, S.; Guldhe, A.; Misra, R.; Rawat, I.; Bux, F. Trends and novel strategies for enhancing lipid accumulation and quality in microalgae. Renew. Sust. Energ. Rev. 2016, 55, 1–16. [Google Scholar] [CrossRef]
- Li, T.; Xu, J.; Gao, B.; Xiang, W.Z.; Zhang, C. Morphology, growth, biochemical composition and photosynthetic performance of Chlorella vulgaris (Trebouxiophyceae) under low and high nitrogen supplies. Algal Res. 2016, 16, 481–491. [Google Scholar] [CrossRef]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High Lipid Induction in Microalgae for Biodiesel Production. Energies 2012, 5, 1532–1553. [Google Scholar] [CrossRef] [Green Version]
- Piligaev, A.V.; Sorokina, K.N.; Samoylova, Y.V.; Parmon, V.N. Production of Microalgal Biomass with High Lipid Content and Their Catalytic Processing Into Biodiesel: A Review. Catal. Ind. 2019, 11, 349–359. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Kiss, R.M.; Priscu, J.C.; Pocock, T.; Gudynaite-Savitch, L.; Huner, N.P.A. Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol. Mol. Biol. Rev. 2006, 70, 222–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Effect of Light Intensity and Quality on Growth Rate and Composition of Chlorella vulgaris. Plants 2020, 9, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varshney, P.; Beardall, J.; Bhattacharya, S.; Wangikar, P.P. Effect of elevated carbon dioxide and nitric oxide on the physiologicalresponses of two green algae, Asterarcys quadricellulare and Chlorella sorokiniana. J. Appl. Phycol. 2020, 32, 189–204. [Google Scholar] [CrossRef]
- Mishra, S.; Liu, Y.-J.; Chen, C.-S.; Yao, D.-J. An Easily Accessible Microfluidic Chip for High-Throughput Microalgae Screening for Biofuel Production. Energies 2021, 14, 1817. [Google Scholar] [CrossRef]
- Takagi, M.; Yoshida, T. Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J. Biosci. Bioeng. 2006, 101, 223–226. [Google Scholar] [CrossRef] [Green Version]
- An, M.; Gao, L.; Zhao, W.; Chen, W.; Li, M. Effects of Nitrogen Forms and Supply Mode on Lipid Production of Microalga Scenedesmus Obliq. Energies 2020, 13, 697. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Han, W.; Li, P.; Miao, X.; Zhong, J. CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour. Technol. 2011, 102, 3071–3076. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, X.; Fan, X.; Han, L.; Zeng, C. Effects of nitrogen source and concentration on growth rate and fatty acid composition of Ellipsoidion sp. (Eustigmatophyta). J. Appl. Phycol. 2001, 13, 463–469. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.; Qin, S.; Zeng, M.; Jiang, Y.; Hu, L.; Xiao, P.; Hao, W.; Hu, Z.; Lei, A.; et al. Growth and lipid accumulation by different nutrients in the microalga Chlamydomonas reinhardtii. Biotechnol. Biofuels 2008, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Vooren, G.V.; Grand, F.L.; Legrand, J.; Cuiné, S.; Peltier, G.; Pruvost, J. Investigation of fatty acids accumulation in Nannochloropsis oculata for biodiesel application. Bioresour. Technol. 2012, 124, 421–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, M.P.; Gautom, T.; Sharma, N. Effect of salinity, pH, light intensity on growth and lipid production of microalgae for bioenergy application. J. Biol. Sci. 2015, 15, 260–267. [Google Scholar] [CrossRef]
- Adams, C.; Godfrey, V.; Wahlen, B.; Seefeldt, L.; Bugbee, B. Understanding precision nitrogen stress to optimize the growth and lipid content tradeoff in oleaginous green microalgae. Biores. Technol. 2013, 131, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Kozieł, W.; Włodarczyk, T. Glony-produkcja biomasy. Acta Agroph. 2011, 17, 105–116. [Google Scholar]
- Makowska, M.; Dziosa, K. Wytwarzanie biomasy mikroalg w warunkach laboratoryjnych. Przem. Chem. 2015, 94, 982–985. [Google Scholar]
- Loera-Quezada, M.M.; Angeles, G.; Olguín, E.J. Effect of irradiance on the cell density, size and lipid accumulation of Neochloris oleoabundans. Rev. Latinoam. Biotechnol. Amb. Algal. 2011, 2, 81–92. [Google Scholar]
- Rendón, L.; Ramírez, M.; Vélez, Y. Microalgas para la Industria Alimenticia; Universidad Pontifica Bolivariana: Medellín, Colombia, 2015. [Google Scholar]
- Escapa, C.; García, A. Eliminación de nutrientes en aguas residuales y biofijación de CO2 mediante el cultivo de microalgas. Sci. Soc. Galicia 2013, 2, 63–76. [Google Scholar]
- Abdel-Raouf, N.; Al-Homaidan, A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [Green Version]
- Markou, G.; Georgakakis, D. Cultivation of filamentous cyanobacteria (blue green algae) in agro-industrial wastes and wastewaters: A review. Appl. Energy 2011, 88, 3389–3401. [Google Scholar] [CrossRef]
- Demirbas, A.; Demirbas, M.F. Importance of Algae Oil as a Source of Biodiesel. Energy Convers. Manag. 2011, 52, 163–170. [Google Scholar] [CrossRef]
- Gao, F.; Li, C.; Yang, Z.H.; Zeng, G.M.; Feng, L.J.; Liu, J.Z.; Liu, M.; Cai, H.W. Continuous microalgae cultivation in aquaculture wastewater by a membrane photobioreactor for biomass production and nutrients removal. Ecol. Eng. 2016, 92, 55–61. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Koniuszy, A.; Gałczyńska, M.; Zając, G.; Szyszlak-Bargłowicz, J. Production of Microalgal Biomass Using Aquaculture Wastewater as Growth Medium. Water 2019, 12, 106. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.; Álvarez-Díaz, P.D.; Arbib, Z.; Garrido-Pérez, C.; Barragán, J.; Perales, J.A. Performance of a flat panel reactor in the continuous culture of microalgae in urban wastewater: Prediction from a batch experiment. Bioresour. Technol. 2013, 127, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lv, J.; Feng, J.; Liu, Q.; Nan, F.; Xie, S. Treatment of real aquaculture wastewater from a fishery utilizing phytoremediation with microalgae. J. Chem. Technol. Biotechnol. 2019, 94, 900–910. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Hu, B.; Min, M.; Chen, P.; Ruan, R.R. Integration of algae cultivation as biodiesel production feedstock with municipal wastewater treatment: Strains screening and significance evaluation of environmental factors. Bioresour. Technol. 2011, 102, 10861–10867. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Koniuszy, A.; Gałczyńska, M. Sustainable Production of Monoraphidium Microalgae Biomass as a Source of Bioenergy. Energies 2020, 13, 5975. [Google Scholar] [CrossRef]
- Fernández-Linares, L.C.; Guerrero-Barajas, C.; Durán-Páramo, E.; Badillo Corona, J.A. Assessment of Chlorella vulgaris and indigenous microalgae biomass with treated wastewater as growth culture medium. Bioresour. Technol. 2017, 244, 400–406. [Google Scholar] [CrossRef]
- Guldhe, A.; Ansari, F.A.; Singh, P.; Bux, F. Heterotrophic cultivation of microalgae using aquaculture wastewater: A biorefinery concept for biomass production and nutrient remediation. Ecol. Eng. 2017, 99, 47–53. [Google Scholar] [CrossRef]
- Mtaki, K.; Kyewalyanga, M.S.; Mtolera, M.S.P. Supplementing wastewater with NPK fertilizer as a cheap source of nutrients in cultivating live food (Chlorella vulgaris). Ann. Microbiol. 2021, 71. [Google Scholar] [CrossRef]
- Tossavainen, M.; Lahti, K.; Edelmann, M.; Eskola, R.; Lampi, A.-M.; Piironen, V.; Korvonen, P.; Ojala, A.; Romantschuk, M. Integrated utilization of microalgae cultured in aquaculture wastewater: Wastewater treatment and production of valuable fatty acids and tocopherols. J. Appl. Phycol. 2019, 31, 1753–1763. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, A.K.H.; Mohsin, K.E. Experimental study for commercial fertilizer NPK (20:20:20+TE N: P: K) in microalgae cultivation at different aeration periods. Iraqi J. Chem. Pet. Eng. 2017, 18, 99–110. [Google Scholar]
- Nayak, M.; Karemore, A.; Sen, R. Performance evaluation of microalgae for concomitant wastewater bioremediation, CO2 biofixation and lipid biosynthesis for biodiesel application. Algal Res. 2016, 16, 216–223. [Google Scholar] [CrossRef]
- Tang, H.; Chen, M.; Simon Ng, K.Y.; Salley, S.O. Continuous microalgae cultivation in a photobioreactor. Biotechnol. Bioeng. 2012, 109, 2468–2474. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, Y.; Xu, J.; Shang, C.; Wang, Z.; Xu, J.; Yuan, Z. Luxury uptake of phosphorus changes the accumulation of starch and lipid in Chlorella sp. under nitrogen depletion. Bioresour. Technol. 2015, 198, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Singh, P.; Guldhe, A.; Bux, F. Microalgal cultivation using aquaculture wastewater: Integrated biomass generation and nutrient remediation. Algal Res. 2017, 21, 169–177. [Google Scholar] [CrossRef]
- Sánchez-García, D.; Resendiz-Isidro, A.; Villegas-Garrido, T.L.; Flores-Ortiz, C.M.; Chávez-Gómez, B.; Cristiani-Urbi, E. Effect of nitrate on lipid production by T. suecica, M. contortum, and C. minutissima. Cent. Eur. J. Biol. 2013, 8, 578–590. [Google Scholar] [CrossRef]
- Do, J.M.; Jo, S.W.; Kim, I.S.; Na, H.; Lee, J.H.; Kim, H.S.; Yoon, H.S. A feasibility study of wastewater treatment using domestic microalgae and analysis of biomass for potential applications. Water 2019, 11, 2294. [Google Scholar] [CrossRef] [Green Version]
- Sacristán de Alva, M.; Luna-Pabello, V.M.; Cadena, E.; Ortiz, E. Green microalgae Scenedesmus acutus grown on municipal wastewater to couple nutrient removal with lipid accumulation for biodiesel production. Bioresour. Technol. 2013, 146, 744–748. [Google Scholar] [CrossRef]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, W.; Min, M.; Ma, X.; Ceria Chandra, Y.T.T.D.; Yiwei, M.; Zheng, H.; Cheng, S.; Griffith, R.; Chen, P.; et al. Growing Chlorella sp. on meat processing wastewater for nutrient removal and biomass production. Bioresour. Technol. 2015, 198, 189–197. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Chinnasamy, S.; Singh, M. Renewable biomass production by mixotrophic algae in the presence of various carbon sources and wastewaters. Appl. Energy 2011, 88, 3425–3431. [Google Scholar] [CrossRef]
- Kim, J.; Lingaraju, B.P.; Rheaume, R.; Lee, J.Y.; Siddiqui, K.F. Removal of ammonia from wastewater effluent by Chlorella vulgaris. Tsinghua Sci. Technol. 2010, 15, 391–396. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Ru, I.T.K.; Sung, Y.Y.; Jusoh, M.; Wahid, M.E.A.; Nagappan, T. Chlorella vulgaris: A perspective on its potential for combining high biomass with high value bioproducts. Appl. Phycol. 2020, 1, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Yeh, K.L.; Aisyah, R.; Lee, D.J.; Chang, J.S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef]
- Wong, Y.K.; Ho, Y.H.; Ho, K.C.; Leung, H.M.; Yung, K.K.L. Growth medium screening for Chlorella vulgaris growth and lipid production. J. Aquac. Mar. Biol. 2017, 6, 00143. [Google Scholar] [CrossRef] [Green Version]
- Geider, R.; La Roche, J. Redfield revisited: Variability of C:N:P in marine microalgae and its biochemical basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Pribyl, P.; Cepak, V.; Zachleder, V. Production of lipids in 10 strains of Chlorella and Parachlorella, and enhanced lipid productivity in Chlorella vulgaris. Appl. Microbiol. Biotechnol. 2012, 94, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, Y.B.; Isik, O.; Uslu, L.; Koc, K.; Durmaz, Y. The effects of nitrogen and phosphorus deficiencies and nitrite addition on the lipid content of Chlorella vulgaris (Chlorophyceae). Afr. J. Biotechnol. 2011, 10, 453–456. [Google Scholar]
- Adenan, N.S.; Yusoff, F.M.; Medipally, S.R.; Shariff, M. Enhancement of lipid production in two marine microalgae under different levels of nitrogen and phosphorus deficiency. J. Environ. Biol. 2016, 37, 669–676. [Google Scholar] [PubMed]
- Chandra, R.; Rohit, M.V.; Swamy, Y.V.; Venkata Mohan, S. Regulatory function of organic carbon supplementation on biodiesel production during growth and nutrient stress phases of mixotrophic microalgae cultivation. Bioresour. Technol. 2014, 165, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Sarat Chandra, T.; Deepak, R.S.; Maneesh Kumar, M.; Mukherji, S.; Chauhan, V.S.; Sarada, R.; Mudliar, S.N. Evaluation of indigenous fresh water microalga Scenedesmus obtusus for feed and fuel applications: Effect of carbon dioxide, light and nutrient sources on growth and biochemical characteristics. Bioresour. Technol. 2016, 207, 430–439. [Google Scholar] [CrossRef]
- Gong, Y.; Guo, X.; Wan, X.; Liang, Z.; Jiang, M. Triacylglycerol accumulation and change in fatty acid content of four marine oleaginous microalgae under nutrient limitation and at different culture ages. J. Basic Microbiol. 2013, 53, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.H.; Gong, Y.P.; Fang, W.Z.; Bi, Z.C.; Cheng, L.H.; Xu, X.H.; Chen, H.L. Saline wastewater treatment by Chlorella vulgaris with simultaneous algal lipid accumulation triggered by nitrate deficiency. Bioresour. Technol. 2015, 193, 68–75. [Google Scholar] [CrossRef]
- Rai, S.V.; Rajashekhar, M. Effect of pH, salinity and temperature on the growth of six species of marine phytoplankton. J. Algal Biomass Util. 2014, 5, 55–59. [Google Scholar]
- Mirizadeh, S.; Nosrati, M.; Shojaosadati, S.A. Synergistic Effect of Nutrient and Salt Stress on Lipid Productivity of Chlorella vulgaris Through Two-Stage Cultivation. Bioenerg. Res. 2020, 13, 507–517. [Google Scholar] [CrossRef]
- Juneja, A.; Ceballos, R.M.; Murthy, G.S. Effects of Environmental Factors and Nutrient Availability on the Biochemical Composition of Algae for Biofuels Production: A Review. Energies 2013, 6, 4607–4638. [Google Scholar] [CrossRef] [Green Version]
- Delgadillo-Mirquez, L.; Lopes, F.; Taidi, B.; Pareau, D. Nitrogen and phosphate removal from wastewater with a mixed microalgae and bacteria culture. Biotechnol. Rep. 2016, 11, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Lugo, L.A.; Thorarinsdottir, R.I.; Bjornsson, S.; Palsson, O.P.; Skulason, H.; Johannsson, S.; Brynjolfsson, S. Remediation of Aquaculture Wastewater Using the Microalga Chlorella sorokiniana. Water 2020, 12, 3144. [Google Scholar] [CrossRef]
- Scherholz, M.L.; Curtis, W.R. Achieving pH control in microalgal cultures through fed-batch addition of stoichiometrically-balanced growth media. BMC Biotechnol. 2013, 13, 39. [Google Scholar] [CrossRef] [Green Version]
- Ördög, V.; Stirk, W.A.; Bálint, P.; Staden, J.; Lovász, C. Changes in lipid, protein and pigment concentrations in nitrogen-stressed Chlorella minutissima cultures. J. Appl. Phycol. 2012, 24, 907–914. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Ferreira, V.; Sant’Anna, C. Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World J. Microbiol. Biotechnol. 2017, 33, 20. [Google Scholar] [CrossRef] [PubMed]
- Mohsenpour, S.F.; Richards, B.; Willoughby, N. Spectral conversion of light for enhanced microalgae growth rates and photosynthetic pigment production. Bioresour. Technol. 2012, 125, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L.; Ryther, J.J. Studies of marine planktonic diatoms in Cyclotella nana Hustedt and Detonula confervacea Cleve. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Ratha, S.K.; Rao, P.H.; Govindaswamy, K.; Jaswin, R.S.; Lakshmidevi, R.; Bhaskar, S.; Chinnasamy, S. A rapid and reliable method for estimating microalgal biomass using a moisture analyser. J. Appl. Phycol. 2016, 28, 1725–1734. [Google Scholar] [CrossRef]
- Ratomski, P.; Hawrot-Paw, M. Production of Chlorella vulgaris biomass in tubular photobioreactors during different culture conditions. Appl. Sci. 2021, 11, 3106. [Google Scholar] [CrossRef]
- Polish Standards PN-86 C-05560/02. Water and Waste Water. Tests for Chlorophyll in Surface Water, Determination of Chlorophyll a in Planktonic Algae by Spectrophotometric Monochromatic Method with Correction for Pheopigments Alpha; Polish Committee for Standardisation. 1986. Available online: https://sklep.pkn.pl/pn-c-05560-02-1986p.html (accessed on 9 November 2020). (In Polish).
- Shin, H.Y.; Ryub, J.H.; Baeb, S.Y.; Crofcheckc, C.; Crockera, M. Lipid extraction from Scenedesmus sp. microalgae for biodiesel production using hot compressed hexane. Fuel 2014, 130, 66–69. [Google Scholar] [CrossRef] [Green Version]
- Polish Standards PN EN ISO 11905-1. Water Quality-Determination of Nitrogen-Part 1: Method Using Oxidative Digestion with Peroxodisulfate; Polish Committee for Standardization. 2001. Available online: https://sklep.pkn.pl/pn-en-iso-11905-1-2001p.html (accessed on 15 November 2020). (In Polish).








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratomski, P.; Hawrot-Paw, M. Influence of Nutrient-Stress Conditions on Chlorella vulgaris Biomass Production and Lipid Content. Catalysts 2021, 11, 573. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050573
Ratomski P, Hawrot-Paw M. Influence of Nutrient-Stress Conditions on Chlorella vulgaris Biomass Production and Lipid Content. Catalysts. 2021; 11(5):573. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050573
Chicago/Turabian StyleRatomski, Patryk, and Małgorzata Hawrot-Paw. 2021. "Influence of Nutrient-Stress Conditions on Chlorella vulgaris Biomass Production and Lipid Content" Catalysts 11, no. 5: 573. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050573