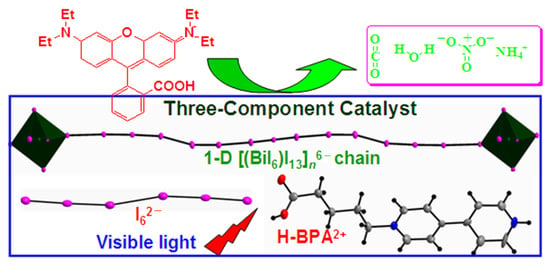
A Three-Component Hybrid Templated by Asymmetric Viologen Exhibiting Visible-Light-Driven Photocatalytic Degradation on Dye Pollutant in Maritime Accident Seawater
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Description
2.2. Absorption Spectrum and Photocurrent Response Behavior
2.3. Photocatalytic Degradation of RhB in Seawater
3. Experimental
3.1. Materials and Methods
3.2. Synthesis
- Synthesis of BPA·Br
- Synthesis of {[(BiI6)I13]·2I3·(H-BPA)4}n (1)
3.3. Photocurrent Measurements
3.4. Photodegradation of RhB
3.5. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.P.; Shang, H.Y.; Liu, X.D.; Bai, Y.J. Fuzzy assessment methodology for emergency response capability in hazardous materials transportation. Int. J. Innov. Comput. Inf. Control 2008, 4, 2689–2696. [Google Scholar]
- Sahoo, C.; Gupta, A.K.; Pal, A. Photocatalytic degradation of Crystal Violet (C.I. Basic Violet 3) on silver ion doped TiO2. Dyes Pigment. 2005, 66, 189–196. [Google Scholar] [CrossRef]
- Maryanti, S.A.; Suciati, S.; Wahyuni, E.S.; Santoso, S.; Wiyasa, I.W.A. Rhodamine B triggers ovarian toxicity through oxidative stress, decreases in the number of follicles, 17B-estradiol level, and thickness of endometrium. Cukurova Med. J. 2014, 39, 451–457. [Google Scholar]
- Feng, Y.; Zhang, Z.; Zhao, Y.; Song, L.; Wang, X.; Yang, S.; Long, Y.; Zhao, C.; Qiu, L. Accelerated rhodamine B removal by enlarged anode electric biological (EAEB) with electro-biological particle electrode (EPE) made from steel converter slag (SCS). Bioresour. Technol. 2019, 283, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Zhang, P.; Dou, Y.; Wu, Y.; Pan, T.; Chu, C.; Cui, P.; Ran, J. Graphene oxide membranes with hierarchical structures used for molecule sieving. Sep. Purif. Technol. 2020, 230, 115879. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, B.; Sang, Y.; Liu, H. Heterostructures construction on TiO2 nanobelts: A powerful tool for building high-performance photocatalysts. Appl. Catal. B 2017, 202, 620–641. [Google Scholar] [CrossRef]
- Robi, S.D.; Jian, Z.; Md, M.; Benjamin, J.C.; Yue, Z.B.; Hareem, K.; Nitu, S.; Ali, Z.; Farjana, H.; Torben, D.; et al. Two dimensional PbMoO4: A photocatalytic material derived from a naturally non-layered crystal. Nano Energy 2018, 49, 237–246. [Google Scholar]
- Hadis, D.; Alireza, N. Increased photocatalytic activity of NiO and ZnO in photodegradation of a model drug aqueous solution: Effect of coupling, supporting, particles size and calcination temperature. J. Hazard. Mater. 2017, 321, 629–638. [Google Scholar]
- Chen, X.; Chen, H.; Guan, J.; Zhen, J.; Sun, Z.; Du, P.; Lu, Y.; Yang, S. A facile mechanochemical route to a covalently bonded graphitic carbon nitride (g-C3N4) and fullerene hybrid toward enhanced visible light photocatalytic hydrogen production. Nanoscale 2017, 9, 5615–5623. [Google Scholar] [CrossRef]
- Wang, D.H.; Lin, X.Y.; Wang, Y.K.; Zhang, W.T.; Song, K.Y.; Lin, H.; Li, H.H.; Chen, Z.R. A new iodiplumbate-based hybird constructed from asymmetric viologen and polyiodides: Structure, properties and photocatalytic aactivity for the degradation of organic dye. Chin. J. Struct. Chem. 2017, 36, 2000–2006. [Google Scholar]
- Li, J.W.; Jiang, C.C. Iodoplumbate(II)-based hybrid templated by 1,4-diazabicyclo[2.2.2]octane derivative: Structure, photocurrent response behavior and photocatalytic activity for the degradation of organic dye. Chin. J. Struct. Chem. 2018, 37, 1635–1644. [Google Scholar]
- Li, H.H.; Zeng, X.H.; Wu, H.Y.; Jie, X.; Zheng, S.T.; Chen, Z.R. Incorporating guest molecules into honeycomb structures constructed from uranium(VI)-polycarboxylates: Structural diversities and photocatalytic activities for the degradation of organic dye. Cryst. Growth Des. 2015, 15, 10–13. [Google Scholar] [CrossRef]
- Song, K.Y.; Zhao, L.M.; Zhang, W.T.; Li, Y.; Li, H.H.; Chen, Z.R. Two-dimensional silver-thiocyanate layers drected by viologens: Structural transformations upon low pressure stimuli, piezochromic luminescence, photocurrent responses, and photocatalytic properties. Cryst. Growth Des. 2019, 19, 177–192. [Google Scholar] [CrossRef]
- Zhao, L.M.; Zhang, W.T.; Song, K.Y.; Wu, Q.Q.; Li, Y.; Li, H.H.; Chen, Z.R. Lead-carboxylate/polyiodide hybrids constructing from halogen bonding and asymmetric viologen: Structures, visible-light-driven photocatalytic properties and enchanced photocurrent responses. CrystEngComm 2018, 20, 2245–2252. [Google Scholar] [CrossRef]
- Adonin, S.A.; Gorokh, I.D.; Novikov, A.S.; Samsonenko, D.G.; Yushina, I.V.; Sokolov, M.N.; Fedin, V.P. Halobismuthates with halopyridinium cations: Appearance or non-appearance of unusual colouring. CrystEngComm 2018, 20, 7766–7772. [Google Scholar] [CrossRef]
- Adonin, S.A.; Gorokh, I.D.; Samsonenko, D.G.; Antonova, O.V.; Korolkov, I.V.; Sokolov, M.N.; Fedin, V.P. Bi(iii) polybromides: A new chapter in coordination chemistry of bismuth. Inorg. Chim. Acta 2018, 32, 5061–5063. [Google Scholar] [CrossRef]
- VenkatRamani, S.; Schrock, R.R.; Hoveyda, A.H.; Müller, P.; Tsay, C. Synthesis of high-oxidation-State Mo-CHX complexes, where X = Cl, CF3, phosphonium, CN. Organometallics 2018, 37, 1641–1644. [Google Scholar] [CrossRef]
- Moyet, M.A.; Kanan, S.M.; Varney, H.M.; Abu-Farha, N.; Gold, D.B.; Lain, W.J.; Pike, R.D.; Patterson, H.H. Synthesis and characterization of (RPh3P)3[Bi3I12] (R = Me, Ph) iodobismuthate complexes for photocatalyticdegradation of organic pollutants. Res. Chem. Intermed. 2019, 45, 5919–5933. [Google Scholar] [CrossRef]
- Wang, Q.; Cai, C.; Wang, M.; Guo, Q.; Wang, B.; Luo, W.; Wang, Y.; Zhang, C.; Zhou, L.; Zhang, D.; et al. Efficient photocatalytic degradation of malachite green in seawater by the hybrid of zinc-oxide nanorods grown on three-dimensional (3D) reduced graphene oxide(RGO)/Ni foam. Materials 2018, 11, 1004. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Hu, X.; Qi, Y.; Wang, X.; Li, Z.; Zhao, L.; Liu, S.; Cui, C. Rapid degradation of azo dyes by melt-spun Mg-Zn-Ca metallic glass in artificial seawater. Metals 2017, 7, 485. [Google Scholar] [CrossRef] [Green Version]
- Makita, M.; Harata, A. Photocatalytic decolorization of rhodamine B dye as a model of dissolved organic compounds: Influence of dissolved inorganic chloride salts in seawater of the sea of Japan. Chem. Eng. Process. 2008, 47, 859–863. [Google Scholar] [CrossRef]
- Wu, L.M.; Wu, X.T.; Chen, L. Structural overview and structure–property relationships of iodoplumbate and iodobismuthate. Coord. Chem. Rev. 2009, 253, 2787–2804. [Google Scholar] [CrossRef]
- Garcia, M.D.; Marti-Rujas, J.; Metrangolo, P.; Peinador, C.; Pilati, T.; Resnati, G.; Terraneo, G.; Ursini, M. Dimensional caging of polyiodides: Cation-templated synthesis using bipyridinium salts. CrystEngComm 2011, 13, 4411–4416. [Google Scholar] [CrossRef] [Green Version]
- Bondi, A.J. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Wang, Y.K.; Wu, Y.L.; Lin, X.Y.; Wang, D.H.; Zhang, W.T.; Song, K.Y.; Li, H.H.; Chen, Z.R. Halobismuthate/diphenyliodonium hybrids stabilized by secondary hypervalent I(III)···X interactions: Structures, optical studies and thermochromisms. J. Mol. Struct. 2018, 1151, 81–87. [Google Scholar] [CrossRef]
- Tong, Z.; Pu, S.; Xiao, Q.; Liu, G.; Cui, S. Synthesis and photochromism of a novel water-soluble diarylethene with glucosyltriazolyl groups. Tetrahedron Lett. 2013, 54, 474–477. [Google Scholar] [CrossRef]
- Patel, E.N.; Arthur, R.B.; Nicholas, A.D.; Reinheimer, E.W.; Omary, M.A.; Brichacek, M.; Patterson, H.H. Synthesis, structure and photophysical properties of a 2D network with gold dicyanide donors coordinated to aza[5]helicene viologen acceptors. Dalton Trans. 2019, 48, 10288–10297. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, S.J.; Kong, F.G. Calixarene-protected titanium-oxo clusters and their photocurrent responses and photocatalytic performances. Inorg. Chem. 2021, 60, 5034–5041. [Google Scholar] [CrossRef]
- Li, J.; Yang, F.; Zhou, Q.; Ren, R.; Wu, L.; Lv, Y. A regularly combined magnetic 3D hierarchical Fe3O4/BiOBr heterostructure: Fabrication, visible-light photocatalytic activity and degradation mechanism. J. Colloid Interface Sci. 2019, 546, 139–151. [Google Scholar] [CrossRef]
- Zhou, X.J.; Chen, C.; Ren, C.X.; Sun, J.K.; Zhang, J. Tunable solid-state photoluminescence based on proton-triggered structural transformation of 4,4prime-bipyridinium derivative. J. Mater. Chem. C 2013, 1, 744–750. [Google Scholar] [CrossRef]
- Wendlandt, W.M.; Hecht, H.G. Reflectance Spectroscopy; Interscience: New York, NY, USA, 1966. [Google Scholar]
- Lin, X.Y.; Zhao, L.M.; Wang, D.H.; Wang, Y.K.; Li, M.; Li, H.H.; Chen, Z.R. Structural diversities of squarate-based complexes: Photocurrent responses and thermochromic behaviours enchanced by viologens. Inorg. Chem. Front. 2018, 5, 189–199. [Google Scholar] [CrossRef]
- Bailey, R.A.; Clark, H.M.; Ferris, J.P.; Krause, S.; Strong, R.L. The environmental chemistry of some important elements. Chem. Environ. 2002, 347–416. [Google Scholar] [CrossRef]
- Huang, H.W.; He, Y.; Lin, Z.S.; Kang, L.; Zhang, Y.H. Two novel Bi-Based Borate photocatalysts: Crystal structure, electronic structure, photoelectrochemical properties, and photocatalytic activity under simulated solar light irradiation. J. Phys. Chem. C 2013, 117, 22986–22994. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-97: Program for Crystal Structure Solution; Göttingen University: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97: Program for the Refinement of Crystal Structures; Göttingen University: Göttingen, Germany, 1997. [Google Scholar]









| Bond | Dist. | Bond | Dist. | Dist. | Dist. |
|---|---|---|---|---|---|
| Bi(1)–I(1) | 3.0568(11) | Bi(1)–I(1) a | 3.0568(11) | Bi(1)–I(2) | 3.1094(12) |
| Bi(1)–I(2) a | 3.1094(12) | Bi(1)–I(3) | 3.0761(10) | Bi(1)–I(3) a | 3.0760(10) |
| I(4)–I(5) | 2.970(2) | I(5)–I(6) | 2.888(2) | I(7)–I(8) | 2.9226(17) |
| I(8)–I(9) | 2.9316(19) | I(10)–I(11) | 2.9024(13) | I(9)–I(12) | 3.361(4) |
| I(10)–I(13) | 3.30(3) | I(12)–I(13) | 2.741(2) | I(3)–I(7) | 3.727(3) |
| Angle | (°) | Angle | (°) | Angle | (°) |
| I(1) a–Bi(1)–I(2) a | 87.53(3) | I(1)–Bi(1)–I(2) a | 92.47(3) | I(3) a–Bi(1)–I(2) a | 89.43(3) |
| I(3)–Bi(1)–I(2) a | 90.57(3) | I(1) a–Bi(1)–I(2) | 92.47(3) | I(1)–Bi(1)–I(2) | 87.53(3) |
| I(3) a–Bi(1)–I(2) | 90.57(3) | I(3)–Bi(1)–I(2) | 89.43(3) | I(2) a–Bi(1)–I(2) | 180.0 |
| I(6)–I(5)–I(4) | 176.62(6) | I(7)–I(8)–I(9) | 176.31(6) | I(10)–I(11)–I(10) b | 180.00(4) |
| I(9)–I(12)–I(13) | 164.87(7) | I(11)–I(10)–I(13) | 144.42(5) |
| D–H…A | d(D–H) | d(H…A) | d(D…A) | ∠DHA | Symmetry Codes |
|---|---|---|---|---|---|
| N(1)–H(1N)…I(2) | 0.86 | 2.84 | 3.597(13) | 149 | −1 − x, 2 − y, −z |
| N(3)–H(3N)…I(2) | 0.86 | 2.79 | 3.578(13) | 153 | −1 + x, y, −1 + z |
| C(5)–H(5)…I(6) | 0.93 | 3.04 | 3.84(2) | 146 | −1 − x, 2 − y, −z |
| O(2)–H(2W)…O(3) | 0.82 | 2.10 | 2.640(14) | 123 | −1 − x, 1/2 + y, 1/2 − z |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, X.; Wu, Q.; Huang, X.; Li, H.; Lin, T.; Gao, Y. A Three-Component Hybrid Templated by Asymmetric Viologen Exhibiting Visible-Light-Driven Photocatalytic Degradation on Dye Pollutant in Maritime Accident Seawater. Catalysts 2021, 11, 640. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050640
Zhuang X, Wu Q, Huang X, Li H, Lin T, Gao Y. A Three-Component Hybrid Templated by Asymmetric Viologen Exhibiting Visible-Light-Driven Photocatalytic Degradation on Dye Pollutant in Maritime Accident Seawater. Catalysts. 2021; 11(5):640. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050640
Chicago/Turabian StyleZhuang, Xueqiang, Qiqi Wu, Xihe Huang, Haohong Li, Tianjin Lin, and Yali Gao. 2021. "A Three-Component Hybrid Templated by Asymmetric Viologen Exhibiting Visible-Light-Driven Photocatalytic Degradation on Dye Pollutant in Maritime Accident Seawater" Catalysts 11, no. 5: 640. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11050640