Economic and Ultrafast Photocatalytic Degradation of Orange II Using Ceramic Powders
Abstract
:1. Introduction
2. Experimental Studies
2.1. Xray Diffraction
2.2. Scanning Electron Microscopy (SEM)/Energy Dispersive X-ray Spectroscopy (EDX)
2.3. Vibrational Spectroscopy
2.4. Optical Analysis
2.5. Photocatalytic Performance
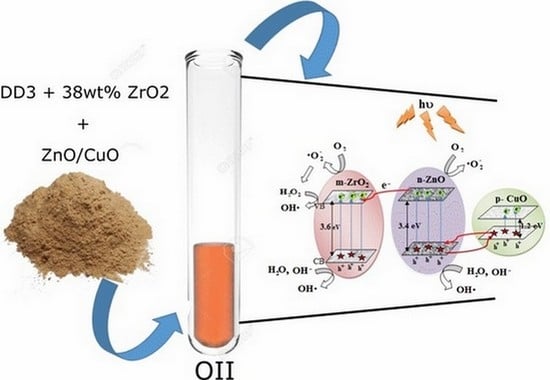
2.6. Mechanism of Photo-Catalysis
2.7. Comparaison with Thin Films
3. Methods
3.1. Materials
3.2. Characterization Techniques
3.3. Sample Preparation
3.3.1. Preparation of Powders
3.3.2. The Photocatalytic Activity Measurement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B

Appendix C

Appendix D

Appendix E
| Phases | Plan (hkl) | 2θ (°) | θ (°) | FHWM β(°) | β(rad) | D (nm) |
|---|---|---|---|---|---|---|
| Mullite | (210) | 26.278 | 13.139 | 0.356 | 0.0062 | 22 |
| Mullite+ +25% Zn+12.5%Cu | (210) | 26.311 | 13.155 | 0.593 | 0.0103 | 13 |
| Mullite +28% Zn+2.8%Cu | (210) | 26.118 | 13.059 | 0.605 | 0.0105 | 13 |
| SiO2 | (101) | 21.680 | 10.84 | 0.261 | 0.0045 | 31 |
| SiO2 +25% Zn+12.5%Cu | (101) | 21.707 | 10.853 | 0.353 | 0.00616 | 22 |
| SiO2 +28% Zn+2.8%Cu | (101) | 21.54 | 10.77 | 0.373 | 0.0065 | 21 |
| Phases | Plan (hkl) | 2θ (°) | θ (°) | FHWM β(°) | β(rad) | D (nm) |
|---|---|---|---|---|---|---|
| Mullite | (121) | 40.821 | 20.41 | 0.382 | 0.0067 | 22 |
| Mullite + 25% Zn + 12.5%Cu | (121) | 40.889 | 20.444 | 0.532 | 0.0093 | 15 |
| Mullite + 28% Zn + 2.8%Cu | (121) | 41.071 | 20.535 | 0.675 | 0.0118 | 12 |
| ZrSiO4 | (200) | 27.017 | 13.508 | 0.188 | 0.0033 | 43 |
| ZrSiO4 + 25% Zn + 12.5%Cu | (200) | 27.068 | 13.534 | 0.468 | 0.0082 | 17 |
| ZrSiO4 + 28% Zn + 2.8%Cu | (200) | 27.217 | 13.608 | 0.656 | 0.0114 | 12 |
| SiO2 | (101) | 21.667 | 10.833 | 0.246 | 0.0043 | 32 |
| SiO2 + 25% Zn + 12.5%Cu | (101) | 21.737 | 10.868 | 0.47 | 0.0082 | 17 |
| SiO2 + 28% Zn + 2.8%Cu | (101) | 21.92 | 10.96 | 0.623 | 0.0109 | 12 |
| ZrO2 | (−111) | 28.196 | 14.098 | 0.209 | 0.0036 | 39 |
| ZrO2 + 25% Zn + 12.5%Cu | (−111) | 28.289 | 14.144 | 0.468 | 0.0082 | 17 |
| ZrO2 + 28% Zn + 2.8%Cu | (−111) | 28.441 | 14.220 | 0.606 | 0.0106 | 13.489 |
References
- Seabra, M.P.; Pires, R.R.; Labrincha, J.A. Ceramic tiles for photodegradation of Orange II solutions. Chem. Eng. J. 2011, 171, 692–702. [Google Scholar] [CrossRef]
- Yang, H.; Xu, S.; Chitwood, D.E.; Wang, Y. Ceramic water filter for point-of-use water treatment in developing countries: Principles, challenges and opportunities. Front. Environ. Sci. Eng. 2020, 14, 79. [Google Scholar] [CrossRef]
- Harabi, A.; Zenikheri, F.; Boudaira, B.; Bouzerara, F.; Guechi, A.; Foughali, L. A new and economic approach to fabricate resistant porous membrane supports using kaolin and CaCO3. J. Eur. Ceram. Soc. 2014, 34, 1329–1340. [Google Scholar] [CrossRef]
- Kouras, N.; Harabi, A.; Bouzerara, F.; Foughali, L.; Policicchio, A.; Stelitano, S.; Galiano, F.; Figoli, A. Macro-porous ceramic supports for membranes prepared from quartz sand and calcite mixtures. J. Eur. Ceram. Soc. 2017, 37, 3159–3165. [Google Scholar] [CrossRef]
- Bouzerara, F.; Boulanacer, S.; Harabi, A. Shaping of microfiltration (MF) ZrO2 membranes using a centrifugal casting method. Ceram. Inter. 2015, 41, 5159–5163. [Google Scholar] [CrossRef]
- Otitojua, T.A.; Okoyeb, P.U.; Chend, G.; Lia, Y.; Okoyec, M.O.; Li, S. Advanced ceramic components: Materials, fabrication, and applications. J. Ind. Eng. Chem. 2020, 85, 34–65. [Google Scholar] [CrossRef]
- Harabi, A.; Karboua, N.; Achour, S. Effect of thickness and orientation of alumina fibrous thermal insulation on microwave heating in a modified domestic 2.45 GHz multi-mode cavity. Int. J. Appl. Ceram. Technol. 2012, 9, 124–132. [Google Scholar] [CrossRef]
- Harabi, E.; Harabi, A.; Mezahi, F.Z.; Zouai, S.; Karboua, N.; Chehalatt, S. Effect of P2O5 on mechanical properties of porous natural hydroxyapatite derived from cortical bovine bones sintered at 1050 °C. Des. Wat. Treat. 2016, 57, 5297–5302. [Google Scholar] [CrossRef]
- Ghouil, B.; Harabi, A.; Bouzerara, F. Elaboration and characterization of ceramic membrane supports from raw materials used in microfiltration. Des. Wat. Treat. 2016, 57, 5241–5245. [Google Scholar] [CrossRef]
- Foughali, L.; Harabi, A.; Barama, S.E.; Bouzerara, F.; Guechi, A.; Boudaira, B. Effect of sodium phosphate additions on mechanical properties of porous Sigue quartz sand. Des. Wat. Treat. 2016, 57, 5286–5291. [Google Scholar] [CrossRef]
- Ghouil, B.; Harabi, A.; Bouzerara, F.; Boudaira, B.; Guechi, A.; Demir, M.M.; Figoli, A. Development and characterization of tubular composite ceramic membranes using natural alumino-silicates for microfiltration applications. Mat. Charact. 2015, 103, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Mdjemem, N.; Harabi, A.; Bouzerara, F.; Foughali, L.; Boudaira, B.; Guechi, A.; Brihi, N. Elaboration and characterization of low cost ceramics microfiltration membranes applied to the sterilization of plant tissue culture media. J. Taiwan Inst. Chem. Eng. 2016, 59, 79–85. [Google Scholar] [CrossRef]
- Harabi, A.; Kasrani, S.; Foughali, L.; Benhassine, M.T.; Kitouni, S. Effect of TiO2 additions on densification and mechanical properties of new mulltifunction resistant porcelains using economic raw materials. Ceram. Inter. 2017, 43, 5547–5556. [Google Scholar] [CrossRef]
- Harabi, E.; Harabi, A.; Foughali, L.; Chehlatt, S.; Zouai, S.; Mezahi, F.Z. Grain growth in sintered natural hydroxyapatite. Acta Phys. Pol. A 2015, 127, 1161–1163. [Google Scholar] [CrossRef]
- Boudaira, B.; Harabi, A.; Bouzerara, F.; Zenikheri, F.; Guechi, A. Preparation and characterization of membrance supports for microfiltration and ultrafiltration using kaolin (DD2) and CaCO3. Des. Wat. Treat. 2016, 57, 5258–5265. [Google Scholar] [CrossRef]
- Chehlatt, S.; Harabi, A.; Oudadesse, H.; Harabi, E. In Vitro Bioactivity Study of Pure Wollastonite Prepared from Local Raw Materials. Acta Phys. Pol. A 2015, 127, 925–927. [Google Scholar] [CrossRef]
- Ma, J.; Wang, K.; Li, L.; Zhang, T.; Kong, Y.; Komarneni, S. Visible-light photocatalytic decolorization of Orange II on Cu2O/ZnO nanocomposites. Ceram. Int. 2015, 41, 2050–2056. [Google Scholar] [CrossRef]
- Siuleiman, S.; Kaneva, N.; Bojinova, A.; Papazova, K.; Apostolov, A.; Dimitrov, D. Photodegradation of Orange II by ZnO and TiO2powders and nanowire ZnO and ZnO/TiO2 thin films. Colloids Surf. A Physicochem. Eng. Aspects 2014, 460, 408–413. [Google Scholar] [CrossRef]
- Mecif, A.; Soro, J.; Harabi, A.; Bonnet, J.P. Preparation of Mullite- and Zircon-Based Ceramics Using Kaolinite and Zirconium Oxide: A. Sintering Study. J. Am. Ceram. Soc. 2010, 93, 1306–1312. [Google Scholar] [CrossRef]
- Witoon, T.; Permsirivanich, T.; Chareonpanich, M. Chitosan-assisted combustion synthesis of CuO–ZnO nanocomposites: Effect of pH and chitosan concentration. Ceram. Int. J. 2013, 39, 3371–3375. [Google Scholar] [CrossRef]
- Ezeigwe, E.R.; Tan, M.T.T.; Khiew, P.S.; Siong, C.W. One-step green synthesis of graphene/ZnO nanocomposites for electrochemical capacitors. Ceram. Int. J. 2015, 41, 715–724. [Google Scholar] [CrossRef]
- Marto, J.; São Marcos, P.; Trindade, T.; Labrincha, J.A. Photocatalytic decolouration of Orange II by ZnO active layers screen-printed on ceramic tiles. J. Hazard. Mater. 2009, 163, 36–42. [Google Scholar] [CrossRef]
- Huang, J.; Dai, Y.; Gu, C.; Sun, Y.; Liu, J. Preparation of porous flower-like CuO/ZnO nanostructures and analysis of their gas-sensing property. J. Alloys Comp. 2013, 575, 115–122. [Google Scholar] [CrossRef]
- Zaman, S. Synthesis of ZnO, CuO and Their Composite Nanostructures for Optoelectronics, Sensing and Catalytic Applications; Linköping University, in Science and Technology: Linköping, Sweden, 2012. [Google Scholar] [CrossRef]
- Kanade, K.G.; Kale, B.B.; Baeg, J.O.; Lee, S.M.; Lee, C.W.; Moon, S.J.; Chang, H. Self-assembled aligned Cu doped ZnO nanoparticles for photocatalytic hydrogen production under visible light irradiation. Mater. Chem. Phys. 2007, 102, 98–104. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Sweena, R.; Wu, J.J.; Anandan, S. Synthesis of CuO-ZnO nanophotocatalys for visible light assisted degradation of a textile dye in aqueous solution. Chem. Eng. J. 2011, 171, 136–140. [Google Scholar] [CrossRef]
- Thaweesaeng, N.; Supankit, S.; Techidheera, W.; Pecharapa, W. Structure properties of as-synthesized Cu-doped ZnO nanopowder synthesized by co-precipitation method. Eng. Procedia 2013, 34, 682–688. [Google Scholar] [CrossRef] [Green Version]
- Shao, B.; Qiu, J.H.; Zhu, K.J.; Cao, Y.; Ji, H.L. Effect of CuO on dielectric and piezoelectric properties of (K0.4425Na0.52Li0.0375) (Nb0.87Ta0.06Sb0.07) O3 ceramics. J. Alloys Compd. 2012, 515, 128–133. [Google Scholar] [CrossRef]
- Jurado, L.T.; Hernández RM, A.; Rangel, E. Sol-Gel synthesis of mullite starting from different inorganic precursors. J. Powder Technol. 2013, 7. [Google Scholar] [CrossRef] [Green Version]
- Vempati, R.K.; Rao, A.; Hess, T.R.; Cocke, D.L.; Lauer, H.V., Jr. Fractionation and characterization of texas lignite class ‘F’ fly ash by XRD, TGA, FTIR and SFM. Cement Concr. Res. 1994, 24, 1153–1164. [Google Scholar] [CrossRef]
- Gougazeha, M.; Buhl, J.-C. Synthesis and characterization of zeolite A bihydrothermal transformation of natural Jordanian kaolin. JAAU Basic Appl. Sci. 2014, 15, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Salje, E.K.H. Infrared spectroscopic analysis of zircon: Radiation damage and the metamict state. J. Phys. Condens. Matter. 2001, 13, 3057–3071. [Google Scholar] [CrossRef]
- Agorku, E.S.; Kuvarega, A.T.; Mamba, B.B.; Pandey, A.C.; Mishra, A.K. Enhanced visible-light photocatalytic activity of multi-elements-doped ZrO2 for degradation of indigo carmine. J. Rare Earths 2015, 33, 498. [Google Scholar] [CrossRef]
- Habibi, M.H.; Karimi, B. Application of impregnation combustion method for fabrication of nanostructure CuO/ZnO composite oxide: XRD, FESEM, DRS and FTIR study. J. Ind. Eng. Chem. 2014, 20, 1566–1570. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Bandara, J.; Herrera, F.G.; Kiwi, J.T.; Pulgarin, C.O. Degradation of concentrated-solutions of non-biodegradable orange-ii by photocatalytic and electrochemical methods. J. Chem. Res. 1998, 234. [Google Scholar] [CrossRef]
- Qiu, R.; Zhang, D.; Mo, Y.; Song, L.; Brewer, E.; Huang, X.; Xiong, Y. Photocatalytic activity of polymer-modified ZnO under visible light irradiation. J. Hazard. Mater. 2008, 156, 80–85. [Google Scholar] [CrossRef]
- Poulios, I.; Micropoulou, E.; Panou, R.; Kostopoulou, E. Photooxidation of eosin Y in the presence of semiconducting oxides. Appl. Catal. B 2003, 41, 345–355. [Google Scholar] [CrossRef]
- Novikov, S.; Timoshcnkov, S. Long-range electrostatic forces on the surfaces ofaluminum oxide and silica oxide’. Adv. Colloid Interface Sci. 2003, 105, 341–353. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, H.; Ma, M.; Yang, Z. Facile synthesis of magnetically recoverable Fe3O4/Al2O3/molecularly imprinted TiO2 nanocomposites and its molecular recognitive photocatalytic degradation of target contaminant. Mol. Catal. A Chem. 2015, 402, 10–16. [Google Scholar] [CrossRef]
- Fu, X.; Tang, W.; Ji, L.; Chen, S. V2O5/Al2O3 composite photocatalyst: Preparation, characterization, and the role of Al2O3. Chem. Eng. J. 2012, 180, 170–177. [Google Scholar] [CrossRef]
- Bansal, P.; Chaudhary, G.R.; Mehta, S.K. Comparative study of catalytic activity of ZrO2 nanoparticles for sonocatalytic and photocatalytic degradation of cationic and anionic dyes. Chem. Eng. J. 2015, 280, 475–485. [Google Scholar] [CrossRef]
- Sheini, F.J.; Singh, J.; Srivasatva, O.N.; Joag, D.S.; More, M.A. Electrochemical synthesis of Cu /ZnO nanocomposite films and their efficient field emission behaviour. Appl. Surf. Sci. 2010, 256, 2110–2114. [Google Scholar] [CrossRef]
- Tan, G.; Huang, J.; Zhang, L.; Ren, H.; Xia, A. An enhanced visible-light-driven photocatalyst: Conduction band control of Bi2WO6 crystallites by Cu ion modification. Ceram. Int. 2014, 40, 11671–11679. [Google Scholar] [CrossRef]
- Saravanana, R.; Karthikeyan, S.; Gupta, V.K.; Sekaran, G.; Narayanane, V.; Stephen, A. Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Mat. Sci. Eng. C 2013, 33, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sakib, A.A.M.; Masum, S.M.; Hoinkis, J.; Islam, R.; Molla, M.A.I. Synthesis of CuO/ZnO Nanocomposites and Their Application in Photodegradation of Toxic Textile Dye. J. Compos. Sci. 2019, 3, 91. [Google Scholar] [CrossRef] [Green Version]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef] [Green Version]
- Katal, R.; Masudy-Panahb, S.; Kongb, E.Y.-J.; Dasineh Khiavic, N.; Davood, M.H.; Farahanid, A.; Gong, X. Nanocrystal-engineered thin CuO film photocatalyst for visible-light-driven photocatalytic degradation of organic pollutant in aqueous solution. Catal. Today 2020, 340, 236–244. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Khedr, M.H.; Ansari, M.S.; Hasan PM, Z.; Abdel-Wahab, M.S.; Farghali, A.A. RF sputtered CuO thin films: Structural, optical and photo-catalytic behavior. Phys. E 2016, 81, 83–90. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Karimi-Shamsabadi, M. Comparison of photocatalytic efficiency of supported CuO onto micro and nano particles of zeolite X in photodecolorization of Methylene blue and Methyl orange aqueous mixture. Appl. Catal. A Gen. 2014, 477, 83–92. [Google Scholar] [CrossRef]
- Fraile, J.M.; Le Jeune, K.; Mayoral, J.A.; Ravasio, N.; Zaccheri, F. CuO/SiO2as a simple, effective and recoverable catalyst for alkylation of indole derivatives with diazocompounds. Org. Biomol. Chem. 2013, 11, 4327. [Google Scholar] [CrossRef] [Green Version]
- Khiavi, N.D.; Katal, R.; Eshkalak, S.K.; Masudy-Panah, S.; Ramakrishna, S.; Jiangyong, H. Visible Light Driven Heterojunction Photocatalyst of CuO–Cu2O Thin Films for Photocatalytic Degradation of Organic Pollutants. Nanomaterials 2019, 9, 1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singha, J.; Mannab, A.K.; Sonia, R.K. Sunlight driven photocatalysis and non-enzymatic glucose sensing performance of cubic structured CuO thin films. Appl. Surf. Sci. 2020, 530, 147258. [Google Scholar] [CrossRef]
- Das, S.; Srivastava, V.C. An overview of the synthesis of CuO-ZnO nanocomposite for environmental and other applications. Nanotechnol. Rev. 2018, 7, 267–282. [Google Scholar] [CrossRef]
- Paz, D.S.; Foletto, E.L.; Bertuol, D.A.; Jahn, S.L.; Collazzo, G.C.; da Silva, S.S.; Chiavone-Filho, O.; do Nascimento, C.A.O. CuO/ZnO coupled oxide films obtained by the electrodeposition technique and their photocatalytic activity in phenol degradation under solar irradiation. Water Sci. Tech. 2013, 68, 5. [Google Scholar] [CrossRef] [PubMed]
- Qamar, M.T.; Aslam, M.; Ismail, I.M.I.; Salah, N.; Hameed, A. Synthesis, Characterization, and Sunlight Mediated Photocatalytic Activity of CuO Coated ZnO for the Removal of Nitrophenols. ACS Appl. Mater. Interfaces 2015, 7, 8757–8769. [Google Scholar] [CrossRef] [PubMed]
- Jothibas, M.; Manoharan, C.; Jeyakumar, S.J.; Praveen, P.; Panneerdoss, I.J. Photocatalytic activity of spray deposited ZrO2 nano-thin films on methylene blue decolouration. J. Mater. Sci. Mater. Electron. 2016, 27, 5851–5859. [Google Scholar] [CrossRef]
- Polisetti, S.; Deshpande, P.A.; Madras, G. Photocatalytic Activity of Combustion Synthesized ZrO2 and ZrO2/TiO2 Mixed Oxides. Ind. Eng. Chem. Res. 2011, 50, 12915–12924. [Google Scholar] [CrossRef]
- Majedi, A.; Davar, F.; Abbasi, A.; Ashrafi, A. Modified Sol–Gel Based Nanostructured Zirconia Thin Film: Preparation, Characterization, Photocatalyst and Corrosion Behavior. J. Inorg. Organomet. Polym. 2016, 26, 932–942. [Google Scholar] [CrossRef]
- Bouras, D.; Mecif, A.; Barillé, R.; Harabi, A.; Rasheed, M.; Mahdjoub, A.; Zaabat, M. Cu:ZnO deposited on porous ceramic substrates by a simple thermal method for photocatalytic application. Ceram. Int. 2018, 44, 21546–21555. [Google Scholar] [CrossRef] [Green Version]
- Ajmal, A.; Riffat, I.M.; Malik, N.; Idrissc, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]








| Material | Powder | Thin Film |
|---|---|---|
| DD3Z + 50 wt% ZnO | 98.9% of a OII (15 min) | |
| DD3 + 50 wt% ZnO | 98.9% of a OII (30 min) | |
| DD3 + 5.37 wt% CuO + 14.28 wt% ZnO | 82.4% (30 min) | |
| DD3Z + 14.28 wt% ZnO + 14.28 wt% ZnO | 93.6% (15 min) | |
| co-precipitation technique 28 wt% ZnO and 2.8 wt% CuO | ||
| DD3 + 28 wt% ZnO and 2.8 wt% CuO | 84% (150 min) | |
| DD3Z + 28 wt% ZnO and 2.8 wt% CuO | 99.6% (45 min) | |
| CuO | Methylene Blue (MB) minimum 2 ppm after 200 min | |
| CuO/zeoliteX [52], CuO/SiO2 [53], heterojunction CuO–Cu2O thin film | Methylene Blue (MB) minimum 2 ppm after 200 min | |
| CuO/ZnO composite oxide thin films | 90% (120 min) | |
| ZnO with CuO to form CuO@ZnO core−shell | nitrophenols (180 min) | |
| ZrO2/TiO2 mixed oxides [58] | 80% degradation of a dye was observed in 2 h | |
| ZrO2 nano-thin films [59] | MB dye 72.13% (180 min) | |
| ZrO2 calcined at 500 °C | 85% methyl orange (60 min) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouras, D.; Mecif, A.; Harabi, A.; Barillé, R.; hakim Mahdjoub, A.; Zaabat, M. Economic and Ultrafast Photocatalytic Degradation of Orange II Using Ceramic Powders. Catalysts 2021, 11, 733. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11060733
Bouras D, Mecif A, Harabi A, Barillé R, hakim Mahdjoub A, Zaabat M. Economic and Ultrafast Photocatalytic Degradation of Orange II Using Ceramic Powders. Catalysts. 2021; 11(6):733. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11060733
Chicago/Turabian StyleBouras, Dikra, Abla Mecif, Abdelhamid Harabi, Régis Barillé, Abdel hakim Mahdjoub, and Mourad Zaabat. 2021. "Economic and Ultrafast Photocatalytic Degradation of Orange II Using Ceramic Powders" Catalysts 11, no. 6: 733. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11060733