Enhanced Photocatalytic Activity of rGO-CuO Nanocomposites for the Degradation of Organic Pollutants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties
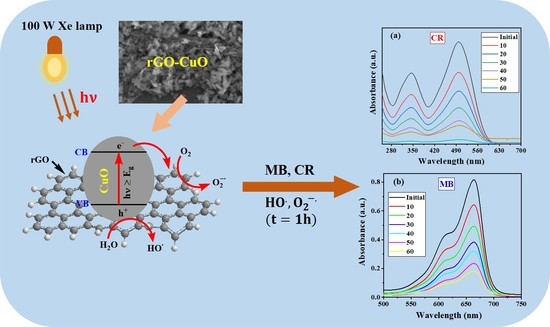
2.2. Photocatalytic Activity of CuO/rGO Nanocomposites
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of CuO/rGO Nanocomposites
3.3. Instrumental Analysis
3.4. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lignier, P.; Bellabarba, R.; Tooze, R.P. Scalable strategies for the synthesis of well-defined copper metal and oxide nanocrystals. Chem. Soc. Rev. 2012, 41, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Saha, S.; Ganguly, A.; Ganguli, A.K. A facile low temperature (350 C) synthesis of Cu2O nanoparticles and their electrocatalytic and photocatalytic properties. RSC Adv. 2014, 4, 12043–12049. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Chen, C.-H.; Huang, M.H. Seed-Mediated Synthesis of Monodispersed Cu2O Nanocubes with Five Different Size Ranges from 40 to 420 nm. Adv. Funct. Mater. 2007, 17, 3773–3780. [Google Scholar] [CrossRef]
- Wang, D.B.; Yu, D.B.; Mo, M.S.; Liu, X.M.; Qian, Y.T. Seed-mediated growth approach to shape-controlled synthesis of Cu2O particles. J. Colloid Interf. Sci. 2003, 261, 565–568. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-graphene composite as a high performance photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Mun, D.-H.; Lee, H.J.; Bae, S.; Kim, T.-W.; Lee, S.H. Photocatalytic decomposition of graphene over a ZnO surface under UV irradiation. Phys. Chem. Chem. Phys. 2015, 17, 15683–15686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Tang, Z.R.; Fu, X.Z.; Xu, Y.J. TiO2−Graphene Nanocomposites for Gas-Phase Photocatalytic Degradation of Volatile Aromatic Pollutant: Is TiO2−Graphene Truly Different from Other TiO2−Carbon Composite Materials? ACS Nano 2010, 4, 7303–7314. [Google Scholar] [CrossRef] [PubMed]
- Sathyamoorthy, R.; Mageshwari, K. Synthesis of hierarchical CuO microspheres: Photocatalytic and antibacterial activities. Phys. E 2013, 47, 157–161. [Google Scholar] [CrossRef]
- Zaman, S.; Zainelabdin, A.; Amin, G.; Nur, O.; Willander, M. Efficient catalytic effect of CuO nanostructures on the degradation of organic dyes. J. Phys. Chem. Solids 2012, 73, 1320–1325. [Google Scholar] [CrossRef] [Green Version]
- Shaabani, B.; Alizadeh-Gheshlaghi, E.; Azizian-Kalandaragh, Y.; Khodayari, A. Preparation of CuO nanopowders and their catalytic activity in photodegradation of Rhodamine-B. Adv. Powder Technol. 2014, 25, 1043–1052. [Google Scholar] [CrossRef]
- Lee, K.H.; Han, S.W.; Kwon, K.Y.; Park, J.B. Systematic analysis of palladium–graphene nanocomposites and their catalytic applications in Sonogashira reaction. J. Colloid Interf. Sci. 2013, 403, 127–133. [Google Scholar] [CrossRef]
- Sun, Y.M.; Hu, X.L.; Luo, W.; Xia, F.F.; Huang, Y.H. Reconstruction of conformal nanoscale MnO on graphene as a high-capacity and long-life anode material for lithium ion batteries. Adv. Funct. Mater. 2013, 23, 2436–2444. [Google Scholar] [CrossRef]
- Zeng, B.; Chen, X.; Luo, Y.; Liu, Q.; Zeng, W. Graphene spheres loaded urchin-like CuxO (x = 1 or 2) for use as a high performance photocatalyst. Ceram. Int. 2014, 40, 5055–5059. [Google Scholar] [CrossRef]
- Zong, M.; Huang, Y.; Wu, H.; Zhao, Y.; Liu, P.; Wang, L. Facile preparation of RGO/Cu2O/Cu composite and its excellent microwave absorption properties. Mater. Lett. 2013, 109, 112–115. [Google Scholar] [CrossRef]
- Wang, M.; Huang, J.; Tong, Z.; Li, W.; Chen, J. Reduced graphene oxide–cuprous oxide composite via facial deposition for photocatalytic dye-degradation. J. Alloys Compd. 2013, 568, 26–35. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Huang, D.; Tronganh, N.; Jiang, Y.; Yu, H.; Ding, N.; Ding, G.; Jiao, Z. Facile synthesis of size-tunable CuO/graphene composites and their high photocatalytic performance. Mater. Res. Bull. 2015, 61, 409–414. [Google Scholar] [CrossRef]
- Weldegebrieal, G.K. Synthesis method, antibacterial and photocatalytic activity of ZnO nanoparticles for azo dyes in wastewater treatment: A review. Inorg. Chem. Commun. 2020, 120, 108140. [Google Scholar] [CrossRef]
- Weldegebrieal, G.K. Photocatalytic and antibacterial activityof CuO nanoparticles biosynthesized using Verbascum thapsus leaves extract. Optik 2020, 204, 164230. [Google Scholar] [CrossRef]
- Dhanalekshmi, S.B.; Priya, R.; Selvi, K.T.; Mangai, K.A.; Weldegebrieal, G.K.; Garg, S.; Sagadevan, S. Microwave-assisted synthesis, characterization and photocatalytic activity of mercury vanadate nanoparticles. Inorg. Chem. Commun. 2021, 131, 108768. [Google Scholar] [CrossRef]
- Chen, Q.W.; Zhang, L.Y.; Chen, G. Facile preparation of graphene-copper nanoparticle composite by in situ chemical reduction for electrochemical sensing of carbohydrates. Anal. Chem. 2012, 84, 171–178. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Gu, Y.-e.; Wang, Z.; Wang, C. One-pot solvothermal synthesis of a Cu2O/Graphene nanocomposite and its application in an electrochemical sensor for dopamine. Microchim. Acta 2011, 173, 103–109. [Google Scholar] [CrossRef]
- Shi, Y.; Qian, X.; Zhou, K.; Tang, Q.; Jiang, S.; Wang, B.; Wang, B.; Yu, B.; Hu, Y.; Yuen, R.K.K. CuO/graphene nanohybrids: Preparation and enhancement on thermal stability and smoke suppression of polypropylene. Ind. Eng. Chem. Res. 2013, 52, 13654–13660. [Google Scholar] [CrossRef]
- Chen, W.; Fan, Z.; Lai, Z. Synthesis of core–shell heterostructured Cu/Cu2O nanowires monitored by in situ XRD as efficient visible-light photocatalysts. J. Mater. Chem. A 2013, 1, 13862–13868. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Yang, L.; Wu, Y. Fabrication of a graphene–cuprous oxide composite. J. Solid State Chem. 2009, 182, 2486–2490. [Google Scholar] [CrossRef]
- Sagadevan, S.; Chowdhury, Z.Z.; Johan, M.R.; Aziz, F.A.; Salleh, E.M.; Hawa, A.; Rafque, R.F. A one-step facile route synthesis of copper oxide/reduced graphene oxide nanocomposite for supercapacitor applications. J. Exp. Nanosci. 2018, 13, 284–296. [Google Scholar] [CrossRef] [Green Version]
- Nafey, A.; Addad, A.; Sieber, B.; Chastanet, G.; Barras, A.; Szunerits, S.; Boukherroub, R. Reduced graphene oxide decorated with Co3O4 nanoparticles (rGO-Co3O4) nanocomposite: A reusable catalyst for highly efficient reduction of 4-nitrophenol, and Cr(VI) and dye removal from aqueous solutions. Chem. Eng. J. 2017, 322, 375–384. [Google Scholar] [CrossRef]
- Dutta, S.; Das, K.; Chakrabarti, K.; Jana, D.; De, S.; De, S. Highly efficient photocatalytic activity of CuO quantum dot decorated rGO nanocomposites. J. Phys. D 2016, 49, 315107. [Google Scholar] [CrossRef]
- Alves, D.C.; Silva, R.; Voiry, D.; Asefa, T.; Chhowalla, M. Copper nanoparticles stabilized by reduced graphene oxide for CO 2 reduction reaction. Mater. Renew. Sustain. Energy 2015, 4, 1–7. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascón, J.M.D. Graphene oxide dispersions in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Merino, M.J.; Guardia, L.; Paredes, J.; Villar-Rodil, S.; Solís-Fernández, P.; Martínez-Alonso, A.; Tascón, J. Vitamin C is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions. J. Phys. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- Hou, S.; Su, S.; Kasner, M.L.; Shah, P.; Patel, K.; Madarang, C.J. Formation of highly stable dispersions of silane-functionalized reduced graphene oxide. Chem. Phys. Lett. 2010, 501, 68–74. [Google Scholar] [CrossRef]
- Kumar, S.; Ojha, A.K.; Bhorolua, D.; Das, J.; Kumar, A.; Hazarika, A. Facile synthesis of CuO nanowires and Cu2O nanospheres grown on rGO surface and exploiting its photocatalytic, antibacterial and supercapacitive properties. Phys. B Condens. Matter 2019, 558, 74–81. [Google Scholar] [CrossRef]
- Mousset, E.; Oturan, N.; Oturan, M.A. An unprecedented route of OH radical reactivity evidenced by an electrocatalytical process: Ipso-substitution with perhalogenocarbon compounds. Appl. Catal. B Environ. 2018, 226, 135–146. [Google Scholar] [CrossRef]
- Botsa, S.M.; Basavaiah, K. Removal of Nitrophenols from wastewater by monoclinic CuO/RGO nanocomposite. Nanotechnol. Environ. Eng. 2018, 4, 1–7. [Google Scholar] [CrossRef]
- Siddique, S.; Zain-ul-Abdin; Waseem, M.; Naseem, T.; Bibi, A.; Hafeez, M.; Din, S.U.; Haq, S.; Qureshi, S. Photo-Catalytic and Anti-microbial Activities of rGO/CuO Nanocomposite. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1359–1372. [Google Scholar] [CrossRef]
- Ravi, K.; Mohan, B.S.; Sree, G.S.; Raju, I.M.; Basavaiah, K.; Rao, B.V. ZnO/RGO nanocomposite via hydrothermal route for photocatalytic degradation of dyes in presence of visible light. Int. J. Chem. Stud. 2018, 6, 20–26. [Google Scholar]
- Aragaw, B.A.; Dagnaw, A. Copper/reduced graphene oxide nanocomposite for high performance photocatalytic methylene blue dye degradation. Ethiop. J. Sci. Technol. 2019, 12, 125–137. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO 2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Li, B.; Liu, T.; Hu, L.; Wang, Y. A facile one-pot synthesis of Cu2O/RGO nanocomposite for removal of organic pollutant. J. Phys. Chem. Solids 2013, 74, 635–640. [Google Scholar] [CrossRef]
- Ramesha, G.K.; Kumara, A.V.; Muralidhara, H.B.; Sampath, S. Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes. J. Colloid Interf. Sci. 2011, 361, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-T.; Chen, S.; Chang, Y.; Cao, A.; Liu, Y.; Wang, H. Removal of methylene blue from aqueous solution by graphene oxide. J. Colloid Interf. Sci. 2011, 359, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Y.; Du, Q.; Sun, J.; Jiao, Y.; Yang, G.; Wang, Z.; Xia, Y.; Zhang, W.; Wang, K.; et al. Adsorption of methylene blue from aqueous solution by graphene. Colloid. Surf. B 2012, 90, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Sagadevan, S.; Lett, J.A.; Weldegebrieal, G.K.; Oh, W.C.; Alshahateet, S.F.; Fatimah, I.; Fatimah, I.; Mohammad, F.; Al-Lohedan, H.A.; Paiman, S.; et al. Enhanced gas sensing and photocatalytic activity of reduced graphene oxide loaded TiO2 nanoparticles. Chem. Phys. Lett. 2021, 780, 138897. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.-Y.; Rooke, J.; van Tendeloo, G.; Su, B.-L. Ultralong Cu(OH)2 and CuO nanowire bundles: PEG200-directed crystal growth for enhanced photocatalytic performance. J. Colloid Interf. Sci. 2010, 348, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Hagfeldt, A.; Gratzel, M. Light-induced redox reactions in nanocrystalline systems. Chem. Rev. 1995, 95, 49–68. [Google Scholar] [CrossRef]








| Dye | The Structural Formula (Molecular Mass (g/mol), Ionicity) | Toxicity | Ref. | |
|---|---|---|---|---|
| CR |  (696.7, anionic) | 497 | Carcinogenic and mutagenic | [17] |
| MB |  (373.9, cationic) | 664 | Anemia, jaundice, dizziness, tissue necrosis, gastrointestinal problems, eye problem in humans | [18,19] |
| S/N | Dye | D (%) | R2 | |||
|---|---|---|---|---|---|---|
| 1 | CR | 95.6 | 60 | 0.0459 | 15.1 | 0.91 |
| 2 | MB | 77.7 | 60 | 0.0248 | 27.9 | 0.998 |
| 3 | MB | 92.8 | 90 | 0.0275 | 25.2 | 0.98 |
| Catalyst | Pollutant | Conditions | D (%) | Ref. |
|---|---|---|---|---|
| CuO/rGO | 2-Nitrophenol | 2-Nitrophenol conc. = 10 ppm, catalyst dose = 30 mg, 400 W metal halide lamp, t = 3 h | 100 | [34] |
| rGO/CuO | 4-Nitrophenol | 4-Nitrophenol conc. = 0.253 ppm, catalyst dose = 10 mg, t = 240 min. under UV light | 81 | [35] |
| 50%rGO/CuO | Methylene blue | MB conc. = 10 ppm, catalyst dose = 20 mg, 150 W Xe lamp, 20 mL H2O2 added, t = 1 h | 99 | [27] |
| ZnO/rGO | Congo red | Congo red conc. = 10 ppm, Dye volume = 100 mL, catalyst dose = 50 mg, under visible light for 1 h | 92 | [36] |
| Cu/rGO | Methylene blue | Dye Conc. = 40 ppm, catalyst dose = 20 mg, t = 50 min., | 94 | [37] |
| CuO-rGO | Methylene blue | MB conc. = 2.23 × 10−5 M, V = 50 mL, Catalyst dose = 10 mg using 100 W Xe lamp irradiating for 90 min. | 77.7 | Present work |
| Congo red | CR conc. = 2.23 × 10−5 M, V = 50 mL, Catalyst dose = 10 mg using 100 W Xe lamp irradiating for 90 min. | 95.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagadevan, S.; Lett, J.A.; Weldegebrieal, G.K.; Garg, S.; Oh, W.-C.; Hamizi, N.A.; Johan, M.R. Enhanced Photocatalytic Activity of rGO-CuO Nanocomposites for the Degradation of Organic Pollutants. Catalysts 2021, 11, 1008. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11081008
Sagadevan S, Lett JA, Weldegebrieal GK, Garg S, Oh W-C, Hamizi NA, Johan MR. Enhanced Photocatalytic Activity of rGO-CuO Nanocomposites for the Degradation of Organic Pollutants. Catalysts. 2021; 11(8):1008. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11081008
Chicago/Turabian StyleSagadevan, Suresh, Jayasingh Anita Lett, Getu Kassegn Weldegebrieal, Seema Garg, Won-Chun Oh, Nor Aliya Hamizi, and Mohd Rafie Johan. 2021. "Enhanced Photocatalytic Activity of rGO-CuO Nanocomposites for the Degradation of Organic Pollutants" Catalysts 11, no. 8: 1008. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11081008