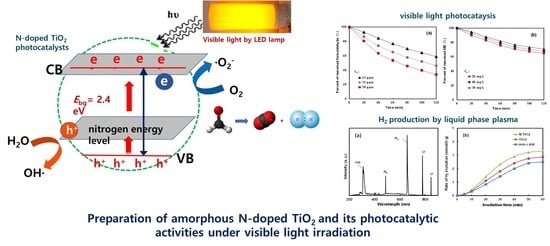
Photocatalytic Properties of Amorphous N-Doped TiO2 Photocatalyst under Visible Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of N/TiO2
2.2. Photocatalytic Activity of the Amorphous N/TiO2 under Visible Light Irradiation
3. Materials and Methods
3.1. Preparation of Amorphous N/TiO2
3.2. Characterization of the N/TiO2 Photocatalyst
3.3. Evaluation of Visible Light Photocatalytic Properties of the Amorphous N/TiO2
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Tage, Y. Visible-light photocatalysis in nitrogen-doped titanium oxide. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, H.J.; Jung, K.T.; Kim, H.S.; Shul, Y.G. Photocatalytic activity of metal ion (Fe or W) doped titania. Korean J. Chem. Eng. 2001, 18, 914–918. [Google Scholar] [CrossRef]
- Dong, C.; Song, H.; Zhou, Y.; Dong, C.; Shen, B.; Yang, H.; Matsuoka, M.; Xing, M.; Zhang, L. Sulfur nanoparticles in situ growth on TiO2 mesoporous single crystals with enhanced solar light photocatalytic performance. RSC Adv. 2016, 6, 77863–77869. [Google Scholar] [CrossRef]
- Gonell, F.; Puga, A.V.; Julian-Lopez, B.; Garcia, H. Copper-doped titania photocatalysts for simultaneous reduction of CO2 and production of H2 from aqueous sulfide. Appl. Catal. B Environ. 2018, 180, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for environmental photocatalytic applications: A review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chien, T.E.; Lai, P.C.; Chiang, Y.H.; Li, K.L.; Lin, J.L. TiS2 transformation into S-doped and N-doped TiO2 with visible-light catalytic activity. Appl. Surf. Sci. 2015, 359, 1–6. [Google Scholar] [CrossRef]
- Nakamura, R.; Tanaka, T.; Nakato, Y. Mechanism for visible light responses in anodic photocurrents at N-doped TiO2 film electrode. J. Phys. Chem. B 2004, 108, 10617–10620. [Google Scholar] [CrossRef]
- Park, C.H.; Zhang, S.B.; Wei, S.-H. Origin of p-type doping difficulty in ZnO: The impurity perspective. Phys. Rev. B 2002, 66, 073202. [Google Scholar] [CrossRef]
- Oh, K.; Hwang, D.K. Brief review on the preparation of N-doped TiO2 and its application to photocatalysis. Korean Chem. Eng. Res. 2019, 57, 331–337. [Google Scholar]
- Nolan, N.T.; Synnott, D.W.; Seery, M.K.; Hinder, S.J.; Wassenhoven, A.V.; Pillai, S.C. Effect of N-doping on the photocatalytic activity of sol-gel TiO2. J. Hazard. Mater. 2012, 211–212, 88–94. [Google Scholar] [CrossRef] [Green Version]
- He, T.; Zeng, X.; Rong, S. The controllable synthesis of substitutional and interstitial nitrogen-doped manganese dioxide: The effects of doping sites on enhancing the catalytic activity. J. Mater. Chem. A 2020, 8, 8383–8396. [Google Scholar] [CrossRef]
- Ashkarran, A.A.; Hamidnezhad, H.; Haddadi, H.; Mahmoudi, M. Double-doped TiO2 nanoparticles as an efficient visible-light-active photocatalyst and antibacterial agent under solar simulated light. Appl. Surf. Sci. 2014, 301, 338–345. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, X.; Zhang, X.; Hou, H.; Dai, K.; Huang, Q.; Chen, H. Enhanced visible light photocatalytic activity of TiO2 assisted by organic semiconductors: A structure optimization strategy of conjugated polymers. J. Mater. Chem. A 2018, 6, 153–159. [Google Scholar] [CrossRef]
- Antony, R.P.; Mathews, T.; Ajikumar, P.K.; Krishna, D.N.; Dash, S.; Tyagi, A.K. Electrochemically synthesized visible light absorbing vertically aligned N-doped TiO2 nanotube array films. Matter. Res. Bull. 2012, 47, 4491–4497. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen doped titanium dioxide as visible-light-sensitive photocatalysts: Designs, developments, and prospects. Chem. Rev. 2014, 144, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Cai, L.; Huang, L.; Yu, H.; Wang, H. Preparation of nitrogen-doped titanium dioxide with visible-light photocatalytic activity using a facile hydrothermal method. J. Phys. Chem. Solids 2008, 69, 1657–1664. [Google Scholar] [CrossRef]
- Valentin, C.D.; Finazzi, E.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Paganini, M.C.; Giamello, E. N-doped TiO2: Theory and experiment. Chem. Phys. 2007, 339, 44–56. [Google Scholar] [CrossRef]
- Hexing, L.; Xinyu, Z.; Yuning, H.; Jian, Z. Supercritical preparation of a highly active S-doped TiO2 photocatalyst for methylene blue mineralization. Environ. Sci. Technol. 2007, 41, 4410. [Google Scholar]
- Zhang, J.; Wu, Y.; Xing, M.; Leghari, S.A.K.; Sajjad, S. Development of modified N doped TiO2 photocatalyst with metals, non-metals and metal oxides. Energy Environ. Sci. 2010, 3, 715–726. [Google Scholar] [CrossRef]
- Hu, X.; Qi, Z.; Wang, X.; Kawazoe, N.; Yang, Y. Nonmetal-metal-semiconductor-promoted P/Ag/Ag2O/Ag3PO4/TiO2 photocatalyst with superior photocatalytic activity and stability. J. Mater. Chem. 2015, A3, 17858–17865. [Google Scholar] [CrossRef]
- Yu, J.C.; Jiang, Y.; Zhang, H. Effects of F-doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Hoang, S.; Berglund, S.P.; Hahn, N.T.; Bard, A.J.; Mullins, C.B. Enhancing visible light photo-oxidation of water with TiO2 nanowire arrays via cotreatment with H2 and NH3: Synergistic effects between Ti3+ and N. J. Am. Chem. Soc. 2012, 134, 3659–3662. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.X.; Chen, X.Y. A visible light response TiO2 photocatalyst realized by cationic S-doping and its application for phenol degradation. J. Hazard. Mater. 2008, 152, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Khatim, O.; Amamra, M.; Chhor, K.; Bell, A.M.T.; Novikov, D.; Vrel, D.; Kanaev, A. Amorphous-anatase phase transition in single immobilized TiO2 nanoparticles. Chem. Phys. Lett. 2013, 55, 53–56. [Google Scholar] [CrossRef]
- Yi, C.; Liao, Q.; Deng, W.; Huang, Y.; Mao, J.; Zhang, B.; Wu, G. The preparation of amorphous TiO2 doped with cationic S and its application to the degradation of DCFs under visible light irradiation. Sci. Total Environ. 2019, 684, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.A. A surface science perspective on TiO2 photocatalysis. Surf. Sci. Rep. 2011, 66, 185–297. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Lu, L.; Li, L. The photocatalytic properties of amorphous TiO2 composite films deposited by magnetron sputtering. Res. Chem. Intermed. 2012, 38, 487–498. [Google Scholar] [CrossRef]
- Liang, J.; Wang, N.; Zhang, Q.; Liu, B.; Kong, X.; Wei, C. Exploring the mechanism of a pure and amorphous black-blue TiO2: H thin film as a photo anode in water splitting. Nano Energy 2017, 42, 151–156. [Google Scholar] [CrossRef]
- Yue, L.; Takeshi, S.; Yoshiki, S.; Naoto, K. Hexagonal-close-packed, hierarchical amorphous TiO2 nanocolumn arrays: Transferability, enhanced photocatalytic activity, and super amphiphilicity without UV irradiation. J. Am. Chem. Soc. 2010, 40, 14755–14762. [Google Scholar]
- Jia, T.; Zhang, J.; Wu, J.; Wang, D.; Liu, Q.; Qi, Y.; Hu, B.; He, P.; Pan, W.; Qi, X. Synthesis amorphous TiO2 with oxygen vacancy as carriers transport channels for enhancing photocatalytic activity. Matter. Lett. 2020, 265, 127465. [Google Scholar] [CrossRef]
- Buddee, S.; Wongnawa, S.; Sirimahachai, U.; Puetpaibool, W. Recyclable UV and visible light photocatalytically active amorphous TiO2 doped with M (III) ions (M = Cr and Fe). Mater. Chem. Phys. 2011, 126, 167–177. [Google Scholar] [CrossRef]
- Tasbbihi, M.; Bendyna, J.K.; Notten, P.H.L.; Hintzen, H.T. A short review on photocatalytic degradation of formaldehyde. J. Nanosci. Nanotechnol. 2015, 15, 6386–6396. [Google Scholar] [CrossRef]
- Lee, H.U.; Lee, S.C.; Choi, S.; Son, B.; Lee, S.M.; Kim, H.J.; Lee, J. Efficient visible-light induced photocatalyst on nanoporous nitrogen-doped titanium dioxide catalysts. Chem. Eng. J. 2013, 228, 756–764. [Google Scholar] [CrossRef]
- Barkul, R.P.; Koli, V.B.; Shewale, V.B.; Patil, M.K.; Delekar, S.D. Visible active nanocrystalline N-doped anatase TiO2 particles for photocatalytic mineralization studies. Mater. Chem. Phys. 2016, 173, 42–51. [Google Scholar] [CrossRef]
- Sun, X.; Liu, H.; Dong, J.; Wei, J.; Zhang, Y. Preparation and characterization of Ce/N-Co-doped TiO2 particles for production of H2 by photocatalytic splitting water under visible light. Catal. Lett. 2010, 135, 219–225. [Google Scholar] [CrossRef]
- Yu, J.G.; Yu, H.G.; Cheng, B.; Zhao, X.J.; Yu, J.C.; Ho, W.K. The effect of calcination temperature on the surface microstructure and photocatalytic activity of TiO2 thin films prepared by liquid phase deposition. J. Phys. Chem. B 2003, 107, 13871–13879. [Google Scholar] [CrossRef]
- Ren, L.; Huang, X.T.; Sun, F.L.; He, X. Preparation and characterization of doped TiO2 nano-dandelion. Mater. Lett. 2007, 61, 427–431. [Google Scholar] [CrossRef]
- Wang, J.W.; Zhu, W.; Zhang, Y.Q.; Liu, S.X. An efficient twostep technique for nitrogen-doped titanium dioxide synthesizing: Visible-light-induced photode-composition of methylene blue. J. Phys. Chem. C 2007, 111, 1010–1014. [Google Scholar] [CrossRef]
- Xu, J.H.; Li, J.X.; Dai, W.L.; Cao, Y.; Li, H.X.; Fan, K.N. Simple fabrication of twist-like helix N, S-codoped titania photocatalyst with visible-light response. Appl. Catal. B Environ. 2008, 79, 72–80. [Google Scholar] [CrossRef]
- Tarasov, A.; Minnerkhanov, A.; Trusov, G.; Konstantinova, E.; Zyubin, A.; Zyubina, T.; Sadovnikov, A.; Dobrovosky, Y.; Doodilin, E. Shedding light on aging of N-doped titania photocatalysts. J. Phys. Chem. C 2015, 119, 18663–18670. [Google Scholar] [CrossRef]
- Sun, S.H.; Jung, S.-C. Facile synthesis of bimetallic Ni-Cu nanoparticles using liquid phase plasma method. Korean J. Chem. Eng. 2016, 33, 1075–1079. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Zou, W. Preparation of amorphous sphere-like TiO2 with excellent photocatalytic performance. Mater. Lett. 2019, 254, 54–57. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Kim, S.-C.; Park, Y.-K.; Kim, B.H.; Kim, H.; Lee, W.-J.; Lee, H.; Jung, S.-C. Facile precipitation of tin oxide nanoparticles on graphene sheet by liquid phase plasma method for enhanced electrochemical properties. Korean J. Chem. Eng. 2018, 35, 750–756. [Google Scholar] [CrossRef]
- Lee, W.-J.; Jeong, S.; Lee, H.; Kim, B.-J.; An, K.-H.; Park, Y.-K.; Jung, S.-C. Facile synthesis of iron-ruthenium bimetallic oxide nanoparticles on carbon nanotube composites by liquid phase plasma method for supercapacitor. Korean J. Chem. Eng. 2017, 34, 2993–2998. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, K.-H.; Kim, B.-J.; Park, Y.-K.; Kim, S.-C.; Jung, S.-C. Photocatalytic Properties of Amorphous N-Doped TiO2 Photocatalyst under Visible Light Irradiation. Catalysts 2021, 11, 1010. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11081010
Chung K-H, Kim B-J, Park Y-K, Kim S-C, Jung S-C. Photocatalytic Properties of Amorphous N-Doped TiO2 Photocatalyst under Visible Light Irradiation. Catalysts. 2021; 11(8):1010. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11081010
Chicago/Turabian StyleChung, Kyong-Hwan, Byung-Joo Kim, Young-Kwon Park, Sang-Chai Kim, and Sang-Chul Jung. 2021. "Photocatalytic Properties of Amorphous N-Doped TiO2 Photocatalyst under Visible Light Irradiation" Catalysts 11, no. 8: 1010. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11081010