Immobilization of TiO2 Nanoparticles in Cement for Improved Photocatalytic Reactivity and Treatment of Organic Pollutants
Abstract
:1. Introduction
1.1. Organic Contaminants in Stormwater
1.2. Challenges and Motivation
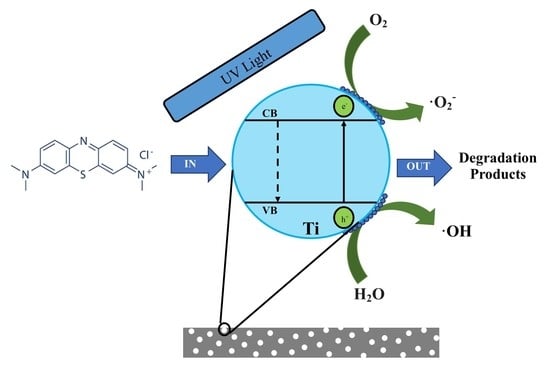
1.3. Photocatalytic Capabilities of TiO2
1.4. Photocatalytic Treatment of Organic Contaminants
1.5. Photocatalytic Concrete
2. Results and Discussion
2.1. Validation of the Functionalization of Ti-MAH
2.2. Validation of Ti-MAH Bonding to Silica within OPC
2.3. Cyclic Photocatalytic Reactivity Testing of TiO2 Versus Ti-MAH in Cement
2.4. Stability of MB+ in Photocatalytic Testing
2.5. Photocatalytic Capabilities of 2Ti-MAH in White Cement Versus Commercially Available Cement
3. Experimental Methods
3.1. Functionalization of Ti-MAH
3.2. Materials and Mixture Proportions
3.3. Mixing and Curing Procedure
3.4. Photocatalytic Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, J.N. Partitioning of Chemical Contaminants in Urban Stormwater. Ph.D. Thesis, University of Otago, Dunedin, New Zealand, 2002. [Google Scholar]
- Foster, G.D.; Roberts, E.C.; Gruessner, B.; Velinsky, D.J. Hydrogeochemistry and transport of organic contaminants in an urban watershed of Chesapeake Bay (USA). Appl. Geochem. 2000, 15, 901–915. [Google Scholar] [CrossRef]
- ITRC (Interstate Technology & Regulatory Council). PFAS Technical and Regulatory Guidance Document and Fact Sheets PFAS-1; Interstate Technology & Regulatory Council, PFAS Team: Washington, DC, USA, 2020; Available online: https://pfas-1.itrcweb.org/ (accessed on 1 July 2021).
- Mahlambi, M.M.; Ngila, C.J.; Mamba, B. Recent Developments in Environmental Photocatalytic Degradation of Organic Pollutants: The Case of Titanium Dioxide Nanoparticles—A Review. J. Nanomater. 2015, 2015, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Spatari, S.; Yu, Z.; Montalto, F.A. Life cycle implications of urban green infrastructure. Environ. Pollut. 2011, 159, 2174–2179. [Google Scholar] [CrossRef]
- Brown, R.A.; Borst, M. Evaluation of Surface Infiltration Testing Procedures in Permeable Pavement Systems. J. Environ. Eng. 2014, 140, 04014001. [Google Scholar] [CrossRef]
- Welker, A.L.; Barbis, J.D.; Jeffers, P.A. A Side-by-Side Comparison of Pervious Concrete and Porous Asphalt. J. Am. Water Resour. Assoc. (JAWRA) 2012, 48, 809–819. [Google Scholar] [CrossRef]
- Asadi, S.; Hassan, M.; Kevern, J.; Rupnow, T.D. Development of Photocatalytic Pervious Concrete Pavement for Air and Storm Water Improvements. Transp. Res. Rec. J. Transp. Res. Board 2012, 2290, 161–167. [Google Scholar] [CrossRef]
- Kayhanian, M.; Li, H.; Harvey, J.T.; Liang, X. Application of permeable pavements in highways for stormwater runoff management and pollution prevention: California research experiences. Int. J. Transp. Sci. Technol. 2019, 8, 358–372. [Google Scholar] [CrossRef]
- Monrose, J.; Tota-Maharaj, K. Permeable Pavements as Low Impact Development Practices for Urban Runoff Mitigation and Sustainable Stormwater Management within the Built Environment for the Caribbean; Conference: 25th Silver Anniversary Annual Caribbean Water and Wastewater Association (CWWA) Conference & Exhibition at: Port of Spain, Trinidad and Tobago, West Indies 2016; pp. 24–28. Available online: https://cwwa.net/publication/permeable-pavements-as-low-impact-development-practices-for-urban-runoff-mitigation-and-sustainable-stormwater-management-within-the-built-environment-for-the-caribbean-presentation/ (accessed on 31 July 2021).
- Spahr, S.; Teixidó, M.; Sedlak, D.L.; Luthy, R.G. Hydrophilic trace organic contaminants in urban stormwater: Occurrence, toxicological relevance, and the need to enhance green stormwater infrastructure. Environ. Sci. Water Res. Technol. 2020, 6, 15–44. [Google Scholar] [CrossRef] [Green Version]
- Environmental Protection Agency (EPA), Office of Water. Preliminary Data Summary of Urban Storm Water Best Management Practices; EPA-821-R-99-012; Environmental Protection Agency: Washington, DC, USA, 1999.
- Todd, G.; Chessin, R.; Colman, J. Toxicological Profile for Total Petroleum Hydrocarbons (TPH); Agency for Toxic Substances and Disease Registry: Atlanta, Georgia, 1999. [Google Scholar]
- Björklund, K. Sources and Fluxes of Organic Contaminants in Urban Runoff. Ph.D. Thesis, Chalmers University of Technology, Göteborg, Sweden, 2011. [Google Scholar]
- Mangiafico, S.S.; Newman, J.; Merhaut, D.J.; Gan, J.; Faber, B.; Wu, L. Nutrients and Pesticides in Stormwater Runoff and Soil Water in Production Nurseries and Citrus and Avocado Groves in California. HortTechnology 2009, 19, 360–367. [Google Scholar] [CrossRef] [Green Version]
- Ellis, J. Pharmaceutical and personal care products (PPCPs) in urban receiving waters. Environ. Pollut. 2006, 144, 184–189. [Google Scholar] [CrossRef]
- Xiao, F.; Simcik, M.F.; Gulliver, J. Perfluoroalkyl acids in urban stormwater runoff: Influence of land use. Water Res. 2012, 46, 6601–6608. [Google Scholar] [CrossRef] [PubMed]
- Codling, G.; Yuan, H.; Jones, P.D.; Giesy, J.P.; Hecker, M. Metals and PFAS in stormwater and surface runoff in a semi-arid Canadian city subject to large variations in temperature among seasons. Environ. Sci. Pollut. Res. 2020, 27, 18232–18241. [Google Scholar] [CrossRef]
- Hassaan, M.A.; Nemr, A.E. Health and Environmental Impacts of Dyes: Mini Review. Am. J. Environ. Sci. Eng. 2017, 1, 64–67. [Google Scholar] [CrossRef]
- Raval, N.P.; Shah, P.U.; Shah, N.K. Malachite green “a cationic dye” and its removal from aqueous solution by adsorption. Appl. Water Sci. 2016, 7, 3407–3445. [Google Scholar] [CrossRef] [Green Version]
- Delnavaz, M.; Ayati, B.; Ganjidoust, H.; Sanjabi, S. Kinetics study of photocatalytic process for treatment of phenolic wastewater by TiO 2 nano powder immobilized on concrete surfaces. Toxicol. Environ. Chem. 2012, 94, 1086–1098. [Google Scholar] [CrossRef]
- Osborn, D.; Hassan, M.; Asadi, S.; White, J. Durability Quantification of TiO2 Surface Coating on Concrete and Asphalt Pavements. J. Mater. Civ. Eng. 2014, 26, 331–337. [Google Scholar] [CrossRef]
- Mills, A.; Hazafy, D.; Parkinson, J.; Tuttle, T.; Hutchings, M.G. Effect of alkali on methylene blue (C.I. Basic Blue 9) and other thiazine dyes. Dye. Pigment. 2011, 88, 149–155. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium Dioxide Photocatalysis. J. Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Khan, M.M.; Adil, S.; Al-Mayouf, A. Metal oxides as photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef] [Green Version]
- Martha, S.; Sahoo, P.C.; Parida, K.M. An overview on visible light responsive metal oxide based photocatalysts for hydrogen energy production. RSC Adv. 2015, 5, 61535–61553. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Grande, F.; Tucci, P. Titanium Dioxide Nanoparticles: A Risk for Human Health? Mini-Rev. Med. Chem. 2016, 16, 762–769. [Google Scholar] [CrossRef]
- Poulopoulos, S.G.; Yerkinova, A.; Ulykbanova, G.; Inglezakis, V.J. Photocatalytic treatment of organic pollutants in a synthetic wastewater using UV light and combinations of TiO2, H2O2 and Fe(III). PLoS ONE 2019, 14, e0216745. [Google Scholar] [CrossRef] [PubMed]
- Khdary, N.H.; Alkhuraiji, W.S.; Sakthivel, T.S.; Khdary, D.N.; Salam, M.A.; Alshihri, S.; Al-Mayman, S.I.; Seal, S. Synthesis of Superior Visible-Light-Driven Nanophotocatalyst Using High Surface Area TiO2 Nanoparticles Decorated with CuxO Particles. Catalysts 2020, 10, 872. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.A.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [Green Version]
- Del Castillo, P.C.H.; Manuel, S.R.; Ruiz, F. An Easy and Efficient Method to Functionalize Titanium Dioxide Nanoparticles with Maleic Anhydride. Soft Nanosci. Lett. 2014, 04, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Umar, M.; Abdul, H. Photocatalytic Degradation of Organic Pollutants in Water; Rashed, M.N., Ed.; IntechOpen: London, UK, 2013; Available online: https://www.intechopen.com/chapters/42060 (accessed on 31 July 2021). [CrossRef] [Green Version]
- Chong, M.N.; Jin, B.; Chow, C.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Yang, G.; Li, C. Electrofiltration of silica nanoparticle-containing wastewater using tubular ceramic membranes. Sep. Purif. Technol. 2007, 58, 159–165. [Google Scholar] [CrossRef]
- Alalm, M.G.; Tawfik, A.; Ookawara, S. Enhancement of photocatalytic activity of TiO2 by immobilization on activated carbon for degradation of pharmaceuticals. J. Environ. Chem. Eng. 2016, 4, 1929–1937. [Google Scholar] [CrossRef]
- Laurance, J. Photocatalytic Treatment of Stormwater Runoff Using Puralytics LilyPad. Honors Baccalaureate of Science in Environmental. Engineering Thesis, Oregon State University, Corvallis, OR, USA, 2016. [Google Scholar]
- Mustapha, S.; Ndamitso, M.M.; Abdulkareem, A.S.; Tijani, J.O.; Shuaib, D.T.; Ajala, A.O.; Mohammed, A.K. Application of TiO2 and ZnO nanoparticles immobilized on clay in wastewater treatment: A review. Appl. Water Sci. 2020, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Doudrick, K.; Monzón, O.; Mangonon, A.; Hristovski, K.; Westerhoff, P. Nitrate Reduction in Water Using Commercial Titanium Dioxide Photocatalysts (P25, P90, and Hombikat UV100). J. Environ. Eng. 2012, 138, 852–861. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Z.; Wang, C.; Li, X.; Wang, C.-C. Photocatalytic degradation of DOM in urban stormwater runoff with TiO2 nanoparticles under UV light irradiation: EEM-PARAFAC analysis and influence of co-existing inorganic ions. Environ. Pollut. 2018, 243, 177–188. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Hsiao, P.-H.; Wei, T.-C.; Chen, T.-C.; Tang, C.-H. Well incorporation of carbon nanodots with silicon nanowire arrays featuring excellent photocatalytic performances. Phys. Chem. Chem. Phys. 2017, 19, 11786–11792. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H.; Chen, K.-Y.; Chen, C.-Y. Solution-processed ZnO/Si based heterostructures with enhanced photocatalytic performance. New J. Chem. 2018, 42, 13797–13802. [Google Scholar] [CrossRef]
- Yang, T.; Yu, D.; Wang, D.; Yang, T.; Li, Z.; Wu, M.; Petru, M.; Crittenden, J. Accelerating Fe(Ⅲ)/Fe(Ⅱ) cycle via Fe(Ⅱ) substitution for enhancing Fenton-like performance of Fe-MOFs. Appl. Catal. B Environ. 2021, 286, 119859. [Google Scholar] [CrossRef]
- Yu, D.; Wang, L.; Yang, T.; Yang, G.; Wang, D.; Ni, H.; Wu, M. Tuning Lewis acidity of iron-based metal-organic frameworks for enhanced catalytic ozonation. Chem. Eng. J. 2021, 404, 127075. [Google Scholar] [CrossRef]
- Yu, D.; Li, L.; Wu, M.; Crittenden, J.C. Enhanced photocatalytic ozonation of organic pollutants using an iron-based metal-organic framework. Appl. Catal. B Environ. 2019, 251, 66–75. [Google Scholar] [CrossRef]
- Cassar, L.; Beeldens, A.; Pimpinelli, N.; Guerrini, G.L. Photocatalysis of cementitious materials. Int. RILEM Symp. Photocatal. Environ. Constr. Mater. 2007, 131–145. [Google Scholar]
- Cackler, T.; Alleman, J.; Kevern, J.; Sikkema, J. Environmental Impact Benefits with “TX Active” Concrete Pavement in Missouri DOT Two-Lift Highway Construction Demonstration; Iowa State University: Ames, IA, USA, 2012. [Google Scholar]
- Yang, L.; Hakki, A.; Wang, F.; Macphee, D.E.; Yang, L.; Hakki, A.; Wang, F.; Macphee, D.E. Photocatalyst efficiencies in concrete technology: The effect of photocatalyst placement. Appl. Catal. B Environ. 2018, 222, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Boonen, E.; Beeldens, A. Recent Photocatalytic Applications for Air Purification in Belgium. Coatings 2014, 4, 553–573. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Burton, M.; Jobson, B.; Haselbach, L. Pervious concrete with titanium dioxide as a photocatalyst compound for a greener urban road environment. Constr. Build. Mater. 2012, 35, 874–883. [Google Scholar] [CrossRef]
- Crain, N.; Mcdonald-Buller, E.; Crain, N.; Juenger, M.; Cros, C.; Terpeluk, A.; Burris, L.; Mcdonald-Buller, E.; Sullivan, D.; Street, G. Laboratory and Field Studies of Photocatalytic NOx and O3 Removal by Coatings on Concrete; The University of Texas at Austin: Austin, TX, USA, 2017. [Google Scholar]
- Samay, G.; Nagy, T.; White, J.L. Grafting maleic anhydride and comonomers onto polyethylene. J. Appl. Polym. Sci. 1995, 56, 1423–1433. [Google Scholar] [CrossRef]
- Layman, A. A Study of the Polarized Infrared Spectrum of Maleic Anhydride. Ph.D. Thesis, Montana State University, Bozeman, MT, USA, 1963. [Google Scholar]
- Beaudoin, J.; Odler, I. (Eds.) Lea’s Chemistry of Cement and Concrete, 5th ed.; Butterworth-Heinemann: Oxford, UK, 2019. [Google Scholar]
- Marchon, D.; Flatt, R.J. (Eds.) Science and Technology of Concrete Admixtures; Elsevier Science Woodhead Publishing: Sawston, UK, 2015. [Google Scholar]
- Nicolas, R.S.; Provis, J.L. The Interfacial Transition Zone in Alkali-Activated Slag Mortars. Front. Mater. 2015, 2, 70. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, H.; Hart, M. Photocatalytic Porous Silica-Based Granular Media for Organic Pollutant Degradation in Industrial Waste-Streams. Catalysts 2021, 11, 258. [Google Scholar] [CrossRef]
- Hsiao, P.-H.; Li, T.-C.; Chen, C.-Y. ZnO/Cu2O/Si Nanowire Arrays as Ternary Heterostructure-Based Photocatalysts with Enhanced Photodegradation Performances. Nanoscale Res. Lett. 2019, 14, 244. [Google Scholar] [CrossRef] [Green Version]
- Grubb, J.; Limaye, H.; Kakade, A. Testing pH of Concrete: Need for a Standard Procedure. Concr. Int. 2007, 29, 78–83. [Google Scholar]
- Hansen, K. Cement Hydration Kinetics; National Precast Concrete Association: Carmel, IN, USA, 2015; Available online: https://precast.org/2016/03/cement-hydration-kinetics/ (accessed on 7 March 2021).
- Lo, Y.; Lee, H. Curing effects on carbonation of concrete using a phenolphthalein indicator and Fourier-transform infrared spectroscopy. Build. Environ. 2002, 37, 507–514. [Google Scholar] [CrossRef]
- ASTM. Standard Specification for Portland Cement; ASTM C150/C150M-16e1; ASTM: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Cao, Q.; Kevern, J.T. Using drinking water treatment waste as a low-cost internal curing agent for concrete. ACI Mater. J. 2015, 112, 69–77. [Google Scholar] [CrossRef]
- International Organization for Standardization. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Photocatalytic Activity of Surfaces in an Aqueous Medium by Degradation of Methylene Blue. 2010. (ISO 10678:2010). Available online: https://www.iso.org/standard/46019.html (accessed on 31 July 2021).
- Milošević, M.; Logar, M.M.; Poharc-Logar, A.V.; Jakšić, N.L. Orientation and Optical Polarized Spectra (380–900 nm) of Methylene Blue Crystals on a Glass Surface. Int. J. Spectrosc. 2013, 2013, 923739. [Google Scholar] [CrossRef] [Green Version]
- Dariani, R.; Esmaeili, A.; Mortezaali, A.; Dehghanpour, S. Photocatalytic reaction and degradation of methylene blue on TiO2 nano-sized particles. Optik 2016, 127, 7143–7154. [Google Scholar] [CrossRef]
- Hou, C.; Hu, B.; Zhu, J. Photocatalytic Degradation of Methylene Blue over TiO2 Pretreated with Varying Concentrations of NaOH. Catalysts 2018, 8, 575. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Jia, Y.; Bu, N.; Wu, J.; Zhen, Q. Photocatalytic degradation of methyl blue using Fe2O3/TiO2 composite ceramics. J. Alloys Compd. 2015, 643, 88–93. [Google Scholar] [CrossRef]
- Xu, C.; Rangaiah, G.; Zhao, X.S. Photocatalytic Degradation of Methylene Blue by Titanium Dioxide: Experimental and Modeling Study. Ind. Eng. Chem. Res. 2014, 53, 14641–14649. [Google Scholar] [CrossRef]

















| Mixture | Portland Cement (g) | TiO2 (g) | 3Ti-MAH (g) | DI Water (g) |
|---|---|---|---|---|
| Control | 14.29 | - | - | 6.93 |
| TiO2 (7%) | 13.29 | 1.00 | - | 6.93 |
| 3Ti-MAH (7%) | 12.96 | - | 1.33 | 6.93 |
| Mixture | Portland Cement (g) | TiO2 (g) | 2Ti-MAH (g) | DI Water (g) |
|---|---|---|---|---|
| Control | 14.29 | - | - | 6.93 |
| 2Ti-MAH (1.5%) | 12.96 | - | 1.33 | 6.93 |
| 2Ti-MAH (7%) | 12.79 | - | 1.5 | 6.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McIntyre, H.M.; Hart, M.L. Immobilization of TiO2 Nanoparticles in Cement for Improved Photocatalytic Reactivity and Treatment of Organic Pollutants. Catalysts 2021, 11, 938. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080938
McIntyre HM, Hart ML. Immobilization of TiO2 Nanoparticles in Cement for Improved Photocatalytic Reactivity and Treatment of Organic Pollutants. Catalysts. 2021; 11(8):938. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080938
Chicago/Turabian StyleMcIntyre, Hannah M., and Megan L. Hart. 2021. "Immobilization of TiO2 Nanoparticles in Cement for Improved Photocatalytic Reactivity and Treatment of Organic Pollutants" Catalysts 11, no. 8: 938. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080938