Conversion of Residual Palm Oil into Green Diesel and Biokerosene Fuels under Sub- and Supercritical Conditions Employing Raney Nickel as Catalyst
Abstract
:1. Introduction
2. Results
2.1. The Residue of Palm Characterization
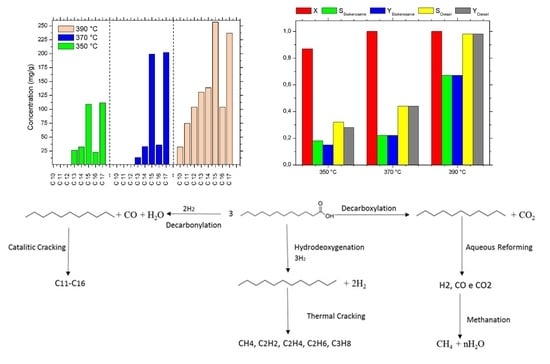
2.2. Effects of Temperature on Hydrothermal Deoxygenation of the Residue of Palm
2.2.1. Effect of Temperature during Hydrothermal Treatment of Palm Waste Using Different Loads of Raney Nickel and after 6 h
2.2.2. Effect of Temperature during Hydrothermal Treatment of Palm Residue Using Different Loads of Raney Nickel and after 3.5 h
2.2.3. Effect of Temperature during Hydrothermal Treatment of Palm Residue Using Different Loads of Raney Nickel and after 1 h
2.3. Reactions Scheme in the Hydrothermal Process of Palm Residue
3. Materials and Methods
3.1. Materials
3.2. Reaction Procedure
3.3. Analysis Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaur, R.; Gera, P.; Jha, M.K.; Bhaskar, T. Reaction parameters effect on hydrothermal liquefaction of castor (Ricinus Communis) residue for energy and valuable hydrocarbons recovery. Renew. Energy 2019, 141, 1026–1041. [Google Scholar] [CrossRef]
- Nanda, S.; Isen, J.; Dalai, A.K.; Kozinski, J.A. Gasification of fruit wastes and agro-food residues in supercritical water. Energy Convers. Manag. 2016, 110, 296–306. [Google Scholar] [CrossRef]
- Dimitriadis, A.; Bezergianni, S. Hydrothermal liquefaction of various biomass and waste feedstocks for biocrude production: A state of the art review. Renew. Sustain. Energy Rev. 2017, 68, 113–125. [Google Scholar] [CrossRef]
- Kim, N.; Vardon, D.R.; Murali, D.; Sharma, B.K.; Strathmann, T.J. Valorization of Waste Lipids through Hydrothermal Catalytic Conversion to Liquid Hydrocarbon Fuels with in Situ Hydrogen Production. ACS Sustain. Chem. Eng. 2015, 4, 1775–1784. [Google Scholar] [CrossRef]
- Araujo, P.H.M. Obtenção de Bioquerosene de Aviação “Drop in” por Pirólise Rápida e Desoxigenação Catalítica a partir do Licuri (Syagrus coronata). Master’s Thesis, Universidade Federal da Paraíba, João Pessoa, Brazil, 2014. [Google Scholar]
- Miao, C.; Marin-Flores, O.; Davidson, S.D.; Li, T.; Dong, T.; Gao, D.; Wang, Y.; Garcia-Pérez, M.; Chen, S. Hydrothermal catalytic deoxygenation of palmitic acid over nickel catalyst. Fuel 2016, 166, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J., Jr.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Kumar, M.; Oyedun, A.O.; Kumar, A. A review on the current status of various hydrothermal technologies on biomass feedstock. Renew. Sustain. Energy Rev. 2018, 81, 1742–1770. [Google Scholar] [CrossRef]
- Minowa, T.; Ogi, T. Hydrogen production from cellulose using a reduced nickel catalyst. Catal. Today 1998, 45, 411–416. [Google Scholar] [CrossRef]
- Yoshida, T.; Oshima, Y.; Matsumura, Y. Gasification of biomass model compounds and real biomass in supercritical water. Biomass Bioenergy 2004, 26, 71–78. [Google Scholar] [CrossRef]
- Sheikhdavoodi, M.J.; Almassi, M.; Ebrahimi-Nik, M.; Kruse, A.; Bahrami, H. Gasification of sugarcane bagasse in supercritical water; evaluation of alkali catalysts for maximum hydrogen production. J. Energy Inst. 2015, 88, 450–458. [Google Scholar] [CrossRef]
- Rase, H.F. Handbook of Commercial Catalysts: Heterogeneous Catalysts; CRC Press: Boca Raton, FL, USA, 2016; ISBN 1482275368. [Google Scholar]
- Studentschnig, A.F.H.; Schober, S.; Mittelbach, M. Conversion of Crude Palm Oil into Hydrocarbons over Commercial Raney Nickel. Energy Fuels 2013, 27, 7480–7484. [Google Scholar] [CrossRef]
- Studentschnig, A.F.; Telser, T.; Schober, S.; Mittelbach, M. Hydrotreating of non-food feedstocks over Raney nickel for the production of synthetic diesel fuel. Biofuels 2016, 7, 279–287. [Google Scholar] [CrossRef]
- de Leeuw, D.W. Water Gasification: Decomposition of Lipids Forming a Substantial Part of Sewage Sludge. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, 2017. [Google Scholar]
- Nanda, S.; Rana, R.; Hunter, H.N.; Fang, Z.; Dalai, A.K.; Kozinski, J.A. Hydrothermal catalytic processing of waste cooking oil for hydrogen-rich syngas production. Chem. Eng. Sci. 2019, 195, 935–945. [Google Scholar] [CrossRef]
- Morgan, T.; Grubb, D.; Santillan-Jimenez, E.; Crocker, M. Conversion of Triglycerides to Hydrocarbons Over Supported Metal Catalysts. Top. Catal. 2010, 53, 820–829. [Google Scholar] [CrossRef]
- Vardon, D.R.; Sharma, B.K.; Jaramillo, H.; Kim, N.; Choe, J.K.; Ciesielski, P.N.; Strathmann, T.J. Hydrothermal catalytic processing of saturated and unsaturated fatty acids to hydrocarbons with glycerol for in situ hydrogen production. Green Chem. 2014, 16, 1507. [Google Scholar] [CrossRef]
- Al Alwan, B.; Salley, S.O.; Ng, K.S. Biofuels production from hydrothermal decarboxylation of oleic acid and soybean oil over Ni-based transition metal carbides supported on Al-SBA-15. Appl. Catal. A Gen. 2015, 498, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Miao, C.; Marin-Flores, O.; Dong, T.; Gao, D.; Wang, Y.; Garcia-Pérez, M.; Chen, S. Hydrothermal Catalytic Deoxygenation of Fatty Acid and Bio-oil with In Situ H2. ACS Sustain. Chem. Eng. 2018, 6, 4521–4530. [Google Scholar] [CrossRef]
- Pinheiro, D.; Amarante, G. Recentes Avanços Em Reações Orgânicas Catalisadas Por NíQUEL. Quim. Nova 2018, 2018, 1033–1054. [Google Scholar] [CrossRef]
- Alenezi, R.; Leeke, G.; Santos, R.; Khan, A. Hydrolysis kinetics of sunflower oil under subcritical water conditions. Chem. Eng. Res. Des. 2009, 87, 867–873. [Google Scholar] [CrossRef]
- Hollak, S.A.W.; Ariëns, M.A.; De Jong, K.P.; Van Es, D.S. Hydrothermal Deoxygenation of Triglycerides over Pd/C aided by In Situ Hydrogen Production from Glycerol Reforming. ChemSusChem 2014, 7, 1057–1062. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, F.; Wu, L.; Wang, C.; Yang, Z. Co-deoxy-liquefaction of biomass and vegetable oil to hydrocarbon oil: Influence of temperature, residence time, and catalyst. Bioresour. Technol. 2011, 102, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huo, X.; Li, Y.; Strathmann, T.J. Catalytic Hydrothermal Decarboxylation and Cracking of Fatty Acids and Lipids over Ru/C. ACS Sustain. Chem. Eng. 2019, 7, 14400–14410. [Google Scholar] [CrossRef]
- Fuentes, E.M. Avaliação de Catalisadores na Reação de Deslocamento de Monóxido de Carbono com Vapor D’água. Master’s Thesis, Universidade Federal da Bahia, Salvador, Brazil, 2006. [Google Scholar]














| Substance | Concentration (mg g−1) | Percentage (%) |
|---|---|---|
| Tetradecanoic acid | 18.25 | 2% |
| Hexadecanoic acid | 374.7 | 34% |
| Linoleic Acid | 110.61 | 10% |
| Trans-Oleic Acid | 258.23 | 23% |
| Oleic acid | 20.91 | 2% |
| Stearic Acid | 74.33 | 7% |
| 2-Monopalmitin | 19.05 | 2% |
| Monoolein | 26.29 | 2% |
| Squalene | Nr | Nr |
| Trilaurin | 137.9 | 18% |
| Trilaurin | 59.75 |
| Temperature (°C) | Catalyst (wt.%) | Time (h) | C10 | C11 | C12 | C13 | C14 | C15 | C16 | C17 |
|---|---|---|---|---|---|---|---|---|---|---|
| 390 | 10 | 6 | 0.00 | 0.00 | 0.00 | 5.47 | 21.65 | 128.32 | 41.17 | 144.32 |
| 390 | 10 | 3.5 | 32.33 | 75.04 | 104.12 | 130.64 | 138.84 | 256.71 | 104.19 | 237.03 |
| 390 | 10 | 1 | 0.00 | 0.00 | 0.00 | 12.16 | 23.44 | 125.35 | 23.49 | 135.83 |
| 390 | 7.5 | 6 | 15.00 | 39.38 | 55.96 | 70.40 | 71.70 | 135.52 | 37.36 | 92.38 |
| 390 | 7.5 | 3.5 | 0.00 | 0.00 | 0.00 | 0.00 | 6.40 | 25.06 | 8.56 | 61.23 |
| 390 | 7.5 | 1 | 0.00 | 0.00 | 53.90 | 71.50 | 76.79 | 159.53 | 57.19 | 154.78 |
| 390 | 5 | 6 | 0.00 | 0.00 | 0.00 | 7.45 | 26.32 | 130.70 | 31.96 | 119.32 |
| 390 | 5 | 3.5 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 85.77 | 0.00 | 91.05 |
| 390 | 5 | 1 | 0.00 | 0.00 | 0.00 | 4.53 | 7.93 | 46.65 | 8.21 | 52.07 |
| 370 | 10 | 6 | 0.00 | 0.00 | 23.78 | 40.32 | 47.03 | 162.48 | 35.71 | 157.09 |
| 370 | 10 | 3.5 | 0.00 | 0.00 | 0.00 | 13.69 | 33.00 | 199.39 | 35.79 | 202.19 |
| 370 | 10 | 1 | 0.00 | 0.00 | 0.00 | 14.78 | 17.57 | 73.00 | 0.00 | 75.81 |
| 370 | 7.5 | 6 | 0.00 | 0.00 | 0.00 | 3.05 | 18.67 | 134.93 | 30.27 | 148.03 |
| 370 | 7.5 | 3.5 | 6.7 | 17.57 | 25.13 | 36.14 | 39.34 | 212.05 | 28.22 | 200.40 |
| 370 | 5 | 6 | 0.00 | 19.59 | 33.42 | 53.11 | 60.70 | 181.66 | 44.35 | 173.96 |
| 370 | 5 | 3.5 | 0.00 | 0.00 | 0.00 | 8.75 | 24.07 | 154.31 | 27.77 | 158.90 |
| 370 | 5 | 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 29.63 | 0.00 | 34.34 |
| 350 | 10 | 6 | 0.00 | 0.00 | 0.00 | 5.42 | 8.60 | 70.58 | 9.59 | 85.97 |
| 350 | 10 | 3.5 | 0.00 | 0.00 | 0.00 | 26.77 | 32.32 | 109.19 | 22.86 | 111.77 |
| 350 | 10 | 1 | 0.00 | 0.00 | 0.00 | 4.28 | 11.03 | 80.79 | 12.80 | 91.71 |
| 350 | 7.5 | 6 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 23.75 | 0.00 | 25.05 |
| 350 | 7.5 | 3.5 | 0.00 | 0.00 | 4.03 | 19.57 | 33.16 | 177.12 | 28.49 | 83.24 |
| 350 | 7.5 | 1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 21.14 | 0.00 | 24.19 |
| 350 | 5 | 6 | 0.00 | 0.00 | 0.00 | 14.97 | 32.27 | 185.72 | 32.38 | 194.38 |
| 350 | 5 | 3.5 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 26.72 | 0.00 | 28.57 |
| 350 | 5 | 1 | 0.00 | 1.42 | 3.51 | 6.90 | 9.14 | 60.88 | 7.98 | 63.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falabella Sousa-Aguiar, E.; Zanon Costa, C.; Peixoto Gimenes Couto, M.A.; de Almeida Azevedo, D.; Filho, J.F.S.d.C. Conversion of Residual Palm Oil into Green Diesel and Biokerosene Fuels under Sub- and Supercritical Conditions Employing Raney Nickel as Catalyst. Catalysts 2021, 11, 995. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080995
Falabella Sousa-Aguiar E, Zanon Costa C, Peixoto Gimenes Couto MA, de Almeida Azevedo D, Filho JFSdC. Conversion of Residual Palm Oil into Green Diesel and Biokerosene Fuels under Sub- and Supercritical Conditions Employing Raney Nickel as Catalyst. Catalysts. 2021; 11(8):995. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080995
Chicago/Turabian StyleFalabella Sousa-Aguiar, Eduardo, Carolina Zanon Costa, Maria Antonieta Peixoto Gimenes Couto, Débora de Almeida Azevedo, and José Faustino Souza de Carvalho Filho. 2021. "Conversion of Residual Palm Oil into Green Diesel and Biokerosene Fuels under Sub- and Supercritical Conditions Employing Raney Nickel as Catalyst" Catalysts 11, no. 8: 995. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11080995