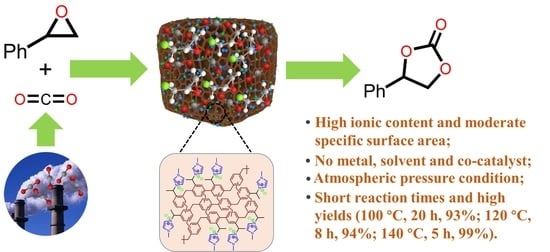
Hypercrosslinked Ionic Polymers with High Ionic Content for Efficient Conversion of Carbon Dioxide into Cyclic Carbonates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Influences of Addition Reaction Conditions on Chloromethyl Content and Specific Surface Area
2.2. The Effects of Ionic Content and Specific Surface Area on Catalytic Activity
2.3. Characterization of Polymers
2.4. Optimization of Cycloaddition Reaction Conditions and the Universality of Catalysts
2.5. Reusability of Catalysts
2.6. Proposed Mechanism
3. Experimental Section
3.1. Materials
3.2. Characterization
3.3. Synthesis of HCP
3.4. Synthesis of HCP-CH2-Cl-X
3.5. Synthesis of [HCP-CH2-Im][Cl]-X
3.6. General Catalytic Procedure for CO2 Cycloaddition to Epoxides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, D.; Ma, L.; Wang, Y.; Zhang, W.; Li, J.; Zhou, Y.; Wang, J. Efficient CO2 enrichment and fixation by engineering micropores of multifunctional hypercrosslinked ionic polymers. Chem. Eng. J. 2020, 390, 124652. [Google Scholar] [CrossRef]
- Xie, Y.; Liang, J.; Fu, Y.; Huang, M.; Xu, X.; Wang, H.; Tu, S.; Li, J. Hypercrosslinked mesoporous poly(ionic liquid)s with high ionic density for efficient CO2 capture and conversion into cyclic carbonates. J. Mater. Chem. A. 2018, 6, 6660–6666. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, F.; Ma, L.; Zhou, Y.; Wang, J. Imidazolium-functionalized ionic hypercrosslinked porous polymers for efficient synthesis of cyclic carbonates from simulated flue gas. ChemSusChem 2020, 13, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015, 6, 5933. [Google Scholar] [CrossRef]
- Ma, D.; Liu, K.; Li, J.; Shi, Z. Bifunctional metal-free porous organic framework heterogeneous catalyst for efficient CO2 conversion under mild and cocatalyst-free conditions. ACS Sustain. Chem. Eng. 2018, 6, 15050–15055. [Google Scholar] [CrossRef]
- Sang, Y.; Huang, J. Benzimidazole-based hyper-cross-linked poly(ionic liquid)s for efficient CO2 capture and conversion. Chem. Eng. J. 2020, 385, 123973. [Google Scholar] [CrossRef]
- Zhang, Y.; El-Sayed, E.M.; Su, K.; Yuan, D.; Han, Z. Facile syntheses of ionic polymers for efficient catalytic conversion of CO2 to cyclic carbonates. J. CO2 Util. 2020, 42, 101301. [Google Scholar] [CrossRef]
- Li, Y.D.; Cui, D.X.; Zhu, J.C.; Huang, P.; Tian, Z.; Jia, Y.Y.; Wang, P.A. Bifunctional phase-transfer catalysts for fixation of CO2 with epoxides under ambient pressure. Green Chem. 2019, 21, 5231–5237. [Google Scholar] [CrossRef]
- Mosteirin, N.F.; Jehanno, C.; Ruiperez, F.; Sardon, H.; Dove, A.P. Rational study of DBU salts for the CO2 insertion into epoxides for the synthesis of cyclic carbonates. ACS Sustain. Chem. Eng. 2019, 7, 10633–10640. [Google Scholar] [CrossRef]
- Xu, X.; Chen, C.; Guo, Z.; North, M.; Whitwood, A.C. Metal-and halide-free catalyst for the synthesis of cyclic carbonates from epoxides and carbon dioxide. ACS Catal. 2019, 9, 1895–1906. [Google Scholar]
- Alves, M.; Grignard, B.; Mereau, R.; Jerome, C.; Tassaing, T.; Detrembleur, C. Organocatalyzed coupling of carbon dioxide with epoxides for the synthesis of cyclic carbonates: Catalyst design and mechanistic studies. Catal. Sci. Technol. 2017, 7, 2651–2684. [Google Scholar] [CrossRef]
- Peng, J.; Yang, H.J.; Geng, Y.; Wei, Z.; Wang, L.; Guo, C.Y. Novel, recyclable supramolecular metal complexes for the synthesis of cyclic carbonates from epoxides and CO2 under solvent-free conditions. J. CO2 Util. 2017, 17, 243–255. [Google Scholar] [CrossRef]
- Deacy, A.C.; Kilpatrick, A.F.R.; Regoutz, A.; Williams, C.K. Understanding metal synergy in heterodinuclear catalysts for the copolymerization of CO2 and epoxides. Nat. Chem. 2020, 12, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Andrea, K.A.; Butler, E.D.; Brown, T.R.; Anderson, T.S.; Jagota, D.; Rose, C.; Lee, E.M.; Goulding, S.D.; Murphy, J.N.; Kerton, F.M.; et al. Iron complexes for cyclic carbonate and polycarbonate formation: Selectivity control from ligand design and metal-center geometry. Inorg. Chem. 2019, 58, 11231–11240. [Google Scholar] [CrossRef]
- Yang, H.Q.; Chen, Z.X. Theoretical investigation on conversion of CO2 with epoxides to cyclic carbonates by bifunctional metal-salen complexes bearing ionic liquid substsituents. Mol. Catal. 2021, 511, 111733. [Google Scholar] [CrossRef]
- Benedito, A.; Acarreta, E.; Gimenez, E. A highly efficient MOF catalyst systems for CO2 conversion to bis-cyclic carbonates as building blocks for NIPHUs (non-isocyanate polyhydroxyurethanes) synthesis. Catalysts 2021, 11, 628. [Google Scholar] [CrossRef]
- Akimana, E.; Wang, J.; Likhanova, N.V.; Chaemchuen, S.; Verpoort, F. MIL-101(Cr) for CO2 conversion into cyclic carbonates under solvent and Co-catalyst free mild reaction conditions. Catalysts 2020, 10, 453. [Google Scholar] [CrossRef]
- Liang, L.; Liu, C.; Jiang, F.; Chen, Q.; Zhang, L.; Xue, H.; Jiang, H.L.; Qian, J.; Yuan, D.; Hong, M. Carbon dioxide capture and conversion by an acid-base resistant metal-organic framework. Nat. Commun. 2017, 8, 1233. [Google Scholar] [CrossRef]
- Pander, M.; Janeta, M.; Bury, W. Quest for an efficient 2-in-1 MOF-based catalytic system for cycloaddition of CO2 to epoxides under mild conditions. ACS Appl. Mater. Interfaces 2021, 13, 8344–8352. [Google Scholar] [CrossRef]
- Abazari, R.; Sanati, S.; Morsali, A.; Kirillov, A.M.; Slawin, A.M.Z.; Carpenter-Warren, C.L. Simultaneous presence of open metal sites and amine groups on a 3D Dy(III)-metal-organic framework catalyst for mild and solvent-free conversion of CO2 to cyclic carbonates. Inorg. Chem. 2021, 60, 2056–2067. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.G.; Han, Z.B. MOF@POP core–shell architecture as synergetic catalyst for high-efficient CO2 fixation without cocatalyst under mild conditions. J. CO2 Util. 2021, 46, 101463. [Google Scholar] [CrossRef]
- Yu, W.; Gu, S.; Fu, Y.; Xiong, S.; Pan, C.; Liu, Y.; Yu, G. Carbazole-decorated covalent triazine frameworks: Novel nonmetal catalysts for carbon dioxide fixation and oxygen reduction reaction. J. Catal. 2018, 362, 1–9. [Google Scholar] [CrossRef]
- Siewniak, A.; Forajter, A.; Szymanska, K. Mesoporous silica-supported ionic liquids as catalysts for styrene carbonate synthesis from CO2. Catalysts 2020, 10, 1363. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y. Facile synthesis of N-rich porous azo-linked frameworks for selective CO2 capture and conversion. Catalysts 2020, 10, 1363. [Google Scholar] [CrossRef]
- Wang, X.; Dong, Q.; Xu, Z.; Wu, Y.; Gao, D.; Xu, Y.; Ye, C.; Wen, Y.; Liu, A.; Long, Z.; et al. Hierarchically nanoporous copolymer with built-in carbene-CO2 adducts as halogen-free heterogeneous organocatalyst towards cycloaddition of carbon dioxide into carbonates. Chem. Eng. J. 2021, 403, 126460. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zuo, K.; Li, Z.; Wang, Y.; Hu, H.; Zeng, C.; Xu, H.; Wang, B.; Gap, Y. Covalent organic frameworks for simultaneous CO2 capture and selective catalytic transformation. Catalysts 2021, 11, 1133. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, J.Y.; Liu, F.; Dai, S. Synthesis of porous polymeric catalysts for the conversion of carbon dioxide. ACS Catal. 2018, 8, 9079–9102. [Google Scholar] [CrossRef]
- Ding, M.; Jiang, H.L. Incorporation of imidazolium-based poly(ionic liquid)s into a metal–organic framework for CO2 capture and conversion. ACS Catal. 2018, 8, 3194–3201. [Google Scholar] [CrossRef]
- Song, H.; Wang, Y.; Xiao, M.; Liu, L.; Liu, Y.; Liu, X.; Gi, H. Design of novel poly(ionic liquids) for the conversion of CO2 to cyclic carbonates under mild conditions without solvent. ACS Sustain. Chem. Eng. 2019, 7, 9489–9497. [Google Scholar] [CrossRef]
- Subramanian, S.; Oppenheim, J.; Kim, D.; Nguyen, T.; Silo, W.; Kim, B.; Goddard, W.; Yavuz, C. Catalytic non-redox carbon dioxide fixation in cyclic carbonates. Chem 2019, 5, 3232–3242. [Google Scholar] [CrossRef]
- Shen, R.; Yan, X.; Guan, Y.J.; Zhu, W.; Li, T.; Liu, X.G.; Li, Y.; Gu, Z.G. One-pot synthesis of a highly porous anionic hypercrosslinked polymer for ultrafast adsorption of organic pollutants. Polym. Chem. 2018, 9, 4724–4732. [Google Scholar] [CrossRef]
- Suo, X.; Huang, Y.; Li, Z.; Pan, H.; Gui, X.; Xing, H. Construction of anion-functionalized hypercrosslinked ionic porous polymers for efficient separation of bioactive molecules. Sci. China Mater. 2021. [Google Scholar] [CrossRef]
- Song, H.; Wang, Y.; Liu, Y.; Chen, L.; Feng, B.; Jin, X.; Zhou, Y.; Huang, T.; Xiao, M.; Huang, F.; et al. Conferring poly(ionic liquid)s with high surface areas for enhanced. ACS Sustain. Chem. Eng. 2021, 9, 2115–2128. [Google Scholar] [CrossRef]
- Wang, J.; Sng, W.; Yi, G.; Zhang, Y. Imidazolium salt-modified porous hypercrosslinked polymers for synergistic CO2 capture and conversion. Chem. Commun. 2015, 51, 12076–12079. [Google Scholar] [CrossRef] [PubMed]
- Pei, B.; Xiang, X.; Liu, T.; Li, D.; Zhao, C.; Qiu, R.; Chen, X.; Lin, J.; Luo, X. Preparation of chloromethylated pitch-based hyper-crosslinked polymers and an immobilized acidic ionic liquid as a catalyst for the synthesis of biodiesel. Catalysts 2019, 9, 963. [Google Scholar] [CrossRef] [Green Version]
- Gan, Y.; Chen, G.; Sang, Y.; Zhou, F.; Man, R.; Huang, J. Oxygen-rich hyper-cross-linked polymers with hierarchical porosity for aniline adsorption. Chem. Eng. J. 2019, 368, 29–36. [Google Scholar] [CrossRef]
- Puthiaraj, P.; Ravi, S.; Yu, K.; Ahn, W.S. CO2 Adsorption and conversion into cyclic carbonates over a porous ZnBr2-grafted N-heterocyclic carbene-based aromatic polymer. Appl. Catal. B Environ. 2019, 251, 195–205. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Patra, P.K.; Hu, D.; Ying, J.Y. Colloidal poly-imidazolium salts and derivatives. Nano Today 2009, 4, 13–20. [Google Scholar] [CrossRef]
- Li, J.; Jia, D.; Guo, Z.; Liu, Y.; Lyu, Y.; Zhou, Y.; Wang, J. Imidazolinium based porous hypercrosslinked ionic polymers for efficient CO2 capture and fixation with epoxides. Green Chem. 2017, 19, 2675–2686. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Xu, J.; Liu, X.; Liu, K.; Tong, M.; Long, Z. Imidazolium-based ionic porous hybrid polymers with POSS-derived silanols for efficient heterogeneous catalytic CO2 conversion under mild conditions. Chem. Eng. J. 2020, 381, 122765. [Google Scholar] [CrossRef]
- Guo, Z.; Jiang, Q.; Shi, Y.; Li, J.; Yang, X.; Hou, W.; Zhou, Y.; Wang, J. Tethering dual hydroxyls into mesoporous poly(ionic liquid)s for chemical fixation of CO2 at ambient conditions: A combined experimental and theoretical study. ACS Catal. 2017, 7, 6770–6780. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Sun, Q.; Fu, Y.; Song, L.; Liang, J.; Xu, X.; Wang, H.; Li, J.; Tu, S.; Lu, X.; et al. Sponge-like quaternary ammonium-based poly(ionic liquid)s for high CO2 capture and efficient cycloaddition under mild conditions. J. Mater. Chem. A 2017, 5, 25594–25600. [Google Scholar] [CrossRef]
- Liu, F.; Huang, K.; Wu, Q.; Dai, S. Solvent-free self-assembly to the synthesis of nitrogen-doped ordered mesoporous polymers for highly selective capture andconversion of CO2. Adv. Mater. 2017, 29, 1700445. [Google Scholar] [CrossRef] [PubMed]
- Ema, T.; Miyazaki, Y.; Shimonishi, J.; Maeda, C.; Hasegawa, J.Y. Bifunctional porphyrin catalysts for the synthesis of cyclic carbonates from epoxides and CO2: Structural optimization and mechanistic study. J. Am. Chem. Soc. 2014, 136, 15270–15279. [Google Scholar] [CrossRef]
- Luo, R.; Zhou, X.; Chen, S.; Li, Y.; Zhou, L.; Ji, H. Highly efficient synthesis of cyclic carbonates from epoxides catalyzed by salen aluminum complexes with built-in “CO2 capture” capability under mild conditions. Green Chem. 2014, 16, 1496–1506. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, D.H.; Qiao, S.; Han, B.H. Synergistic catalysis of ionic liquid-decorated covalent organic frameworks with polyoxometalates for CO2 cycloaddition reaction under mild conditions. Langmuir 2021, 37, 10330–10339. [Google Scholar] [CrossRef]
- Guo, Z.; Cai, X.; Xie, J.; Wang, X.; Zhou, Y.; Wang, J. Hydroxyl-exchanged nanoporous ionic copolymer toward low-temperature cycloaddition of atmospheric carbon dioxide into carbonates. ACS Appl. Mater. Interfaces 2016, 8, 12812–12821. [Google Scholar] [CrossRef]










| Entry | Sample a | IL Content b (mmol g−1) | SBET c (m2 g−1) | Yield d (%) | Sel. d (%) |
|---|---|---|---|---|---|
| 1 | HCP | - | 1153 | 5 | 99 |
| 2 | HCP-CH2-Cl-1 | - | 530 | 7 | 99 |
| 3 | [HCP-CH2-Im][Cl]-1 | 2.10 | 385 | 95 | >99 |
| 4 | [HCP-CH2-Im][Cl]-2 | 0.99 | 510 | 89 | >99 |
| 5 | [HCP-CH2-Im][Cl]-3 | 0.48 | 386 | 74 | 99 |
| 6 | [HCP-CH2-Im][Cl]-4 | 0.85 | 503 | 88 | >99 |
| 7 | [HCP-CH2-Im][Cl]-5 | 1.65 | 183 | 72 | 99 |
| 8 | [HCP-CH2-Im][Cl]-6 | 1.81 | 406 | 84 | >99 |
| 9 | [HCP-CH2-Im][Cl]-7 | 1.26 | 295 | 72 | 99 |
| Entry a | Substrate | Product | Time (h) | Yield b (%) | Sel. b (%) |
|---|---|---|---|---|---|
| 1 |  |  | 4 | 99 | 99 |
| 2 |  |  | 4 | 99 | 99 |
| 3 |  |  | 4 | 99 | >99 |
| 4 |  |  | 5 8 20 | 99 94 (120 °C) 93 (100 °C) | 99 >99 99 |
| 5 |  |  | 6 | 92 | >99 |
| 6 |  |  | 6 | 91 | >99 |
| 7 c |  |  | 6 | 95 c | - |
| 8 |  |  | 6 | 99 | 99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, X.; Pei, B.; Ma, R.; Kong, L.; Gao, X.; He, J.; Luo, X.; Lin, J. Hypercrosslinked Ionic Polymers with High Ionic Content for Efficient Conversion of Carbon Dioxide into Cyclic Carbonates. Catalysts 2022, 12, 62. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12010062
Liao X, Pei B, Ma R, Kong L, Gao X, He J, Luo X, Lin J. Hypercrosslinked Ionic Polymers with High Ionic Content for Efficient Conversion of Carbon Dioxide into Cyclic Carbonates. Catalysts. 2022; 12(1):62. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12010062
Chicago/Turabian StyleLiao, Xu, Baoyou Pei, Ruixun Ma, Lingzheng Kong, Xilin Gao, Jiao He, Xiaoyan Luo, and Jinqing Lin. 2022. "Hypercrosslinked Ionic Polymers with High Ionic Content for Efficient Conversion of Carbon Dioxide into Cyclic Carbonates" Catalysts 12, no. 1: 62. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12010062