Au/Ti Synergistically Modified Supports Based on SiO2 with Different Pore Geometries and Architectures
Abstract
:1. Introduction
2. Results and Discussion
2.1. Properties of Supported Au/Ti Photocatalysts
2.2. Adsorption and Photocatalytic Activity
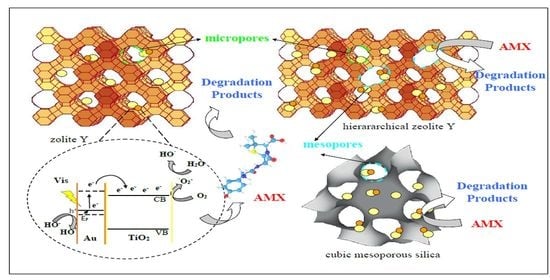
2.3. Proposed Mechanism for the Photocatalytic Degradation of AMX
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Materials
3.3. Characterization of Materials
3.4. Adsorption and Photocatalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoshiiri, K.; Wang, K.; Kowalska, E. TiO2/Au/TiO2 Plasmonic Photocatalysts: The Influence of Titania Matrix and Gold Properties. Inventions 2022, 7, 54. [Google Scholar] [CrossRef]
- Do, T.C.M.V.; Nguyen, D.Q.; Nguyen, K.T.; Le, P.H. TiO2 and Au-TiO2 Nanomaterials for Rapid Photocatalytic Degradation of Antibiotic Residues in Aquaculture Wastewater. Materials 2019, 12, 2434. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Kalwei, M.; Schüth, F.; Chao, K. Gold nanoparticles in SBA-15 showing catalytic activity in CO oxidation. Appl. Catal. A 2003, 254, 289–296. [Google Scholar] [CrossRef]
- Moragues, A.; Puértolas, B.; Mayoral, Á.; Arenal, R.; Hungría, A.B.; Murcia-Mascarós, S.; Taylor, S.H.; Solsona, B.; García, T.; Amorós, P. Understanding the role of Ti-rich domains in the stabilization of gold nanoparticles on mesoporous silica-based catalysts. J. Catal. 2018, 360, 187–200. [Google Scholar] [CrossRef]
- Luna, M.; Gatica, J.M.; Vidal, H.; Mosquera, M.J. Au-TiO2/SiO2 photocatalysts with NOx depolluting activity: Influence of gold particle size and loading. Chem. Eng. J. 2019, 368, 417–427. [Google Scholar] [CrossRef]
- Qi, F.; Wang, C.; Cheng, N.; Liu, P.; Xiao, Y.; Li, F.; Sun, X.; Liu, W.; Guo, S.; Zhao, X.-Z. Improving the performance through SPR effect by employing Au@SiO2 core-shell nanoparticles incorporated TiO2 scaffold in efficient hole transport material free perovskite solar cells. Electrochim. Acta 2018, 282, 10–15. [Google Scholar] [CrossRef]
- Filip, M.; Todorova, S.; Shopska, M.; Ciobanu, M.; Papa, F.; Somacescu, S.; Munteanu, C.; Parvulescu, V. Effects of Ti loading on activity and redox behavior of metals in PtCeTi/KIT-6 catalysts for CH4 and CO oxidation. Catal. Today 2018, 306, 138–144. [Google Scholar] [CrossRef]
- Rizzi, F.; Castaldo, R.; Latronico, T.; Lasala, P.; Gentile, G.; Lavorgna, M.; Striccoli, M.; Agostiano, A.; Comparelli, R.; Depalo, N.; et al. High Surface Area Mesoporous Silica Nanoparticles with Tunable Size in the Sub-Micrometer Regime: Insights on the Size and Porosity Control Mechanisms. Molecules 2021, 26, 4247. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Jung, H.S.; Kim, J.M.; Kang, Y.T. Photocatalytic CO2 conversion on highly ordered mesoporous materials: Comparisons of metal oxides and compound semiconductors. Appl. Catal. B Environ. 2018, 224, 594–601. [Google Scholar] [CrossRef]
- Sun, Z.; Bai, C.; Zheng, S.; Yang, X.; Frost, R.L. A comparative study of different porous amorphous silica mineral ssupported TiO2 catalysts. Appl. Catal. A Gen. 2013, 458, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Vodyankin, A.A.; Vodyankin, O.V. The Effect of Support on the Surface Properties and Photocatalytic Activity of Supported TiO2 Catalysts. Key Eng. Mater. 2016, 670, 224–231. [Google Scholar] [CrossRef]
- Yadav, R.; Amoli, V.; Singh, J.; Tripathi, M.K.; Bhanja, P.; Bhaumik, A.; Sinha, A.K. Plasmonic gold deposited on mesoporous TixSi1−xO2 with isolated silica in lattice: An excellent photocatalyst for photocatalytic conversion of CO2 into methanol under visible light irradiation. J. CO2 Util. 2018, 27, 11–21. [Google Scholar] [CrossRef]
- Mureseanu, M.; Filip, M.; Somacescu, S.; Baran, A.; Carja, G.; Parvulescu, V. Ce, Ti modified MCM-48 mesoporous photocatalysts: Effect of the synthesis route on support and metal ion properties. Appl. Surf. Sci. 2018, 444, 235–342. [Google Scholar] [CrossRef]
- Peng, R.; Zhao, D.; Dimitrijevic, N.M.; Rajh, T.; Koodali, R.T. Room Temperature Synthesis of Ti–MCM-48 and Ti–MCM-41 Mesoporous Materials and Their Performance on Photocatalytic Splitting of Water. J. Phys. Chem. C. 2012, 116, 1605–1613. [Google Scholar] [CrossRef]
- Jianga, C.; Lee, K.Y.; Parlett, C.M.A.; Bayazit, M.K.; Lau, C.C.; Ruan, Q.; Moniz, S.J.A.; Lee, A.F.; Tang, J. Size-controlled TiO2 nanoparticles on porous hosts for enhanced photocatalytic hydrogen production. Appl. Catal. A Gen. 2016, 521, 133–139. [Google Scholar] [CrossRef]
- Petcu, G.; Anghel, E.M.; Somacescu, S.; Preda, S.; Culita, D.; Mocanu, S.; Ciobanu, M.; Parvulescu, V. Hierarchical Zeolite Y Containing Ti and Fe Oxides as Photocatalysts for Degradation of Amoxicillin. J. Nanosci. Nanotechnol. 2020, 20, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-R.; Kim, S. Enhanced Catalytic Oxidation of Toluene over Hierarchical Pt/Y Zeolite. Catalysts 2022, 12, 622. [Google Scholar] [CrossRef]
- Ayati, A.; Ahmadpour, A.; Bamoharram, F.F.; Tanhaei, B.; Manttari, M.; Sillanpaa, M. A review on catalytic applications of Au/TiO2 nanoparticles in the removal of water pollutant. Chemosphere 2014, 107, 163–174. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, X.; Zheng, Z.; Ke, X.; Jaatinen, E.; Zhao, J.; Guo, C.; Xied, T.; Wangd, D. Mechanism of supported gold nanoparticles as photocatalysts under ultraviolet and visible light irradiation. Chem. Commun. 2009, 7524, 7524–7526. [Google Scholar] [CrossRef]
- Tang, K.Y.; Chen, J.X.; Legaspi, E.D.R.; Owh, C.; Lin, M.; Tee, I.S.Y.; Kai, D.; Loh, X.J.; Li, Z.; Regulacio, M.D.; et al. Gold-decorated TiO2 nanofibrous hybrid for improved solar-driven photocatalytic pollutant degradation. Chemosphere 2021, 265, 129114. [Google Scholar] [CrossRef]
- Sacaliuc, E.; Beale, A.M.; Weckhuysen, B.M.; Nijhuis, T.A. Propene epoxidation over Au/Ti-SBA-15 catalysts. J. Catal. 2007, 248, 235–248. [Google Scholar] [CrossRef]
- Gutiérrez, L.F.; Hamoudi, S.; Belkacemi, K. Synthesis of Gold Catalysts Supported on Mesoporous Silica Materials: Recent Developments. Catalysts 2011, 1, 97–154. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, X.; Wang, Y.; Chen, W. An efficient oxidation of cyclohexane over Au@TiO2/MCM-41 catalyst prepared by photocatalytic reduction method using molecular oxygen as oxidant. Catal. Commun. 2014, 46, 228–233. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Z.; Wang, G.; Duan, X.; Qian, G.; Zhou, X. Zeolite crystal size effects of Au/uncalcined TS-1 bifunctional catalysts on direct propylene epoxidation with H2 and O2. Chem. Eng. Sci. 2020, 227, 115907. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, C.; Wu, D.; He, S.; Ren, B. A Simple Method of Preparation of High Silica Zeolite Y and Its Performance in the Catalytic Cracking of Cumene. J. Nanotehnol. 2016, 2016, 1486107. [Google Scholar] [CrossRef]
- Basha, S.; Barr, C.; Keane, D.; Nolan, K.; Morrissey, A.; Oelgemoller, M.; Tobin, J.M. On the adsorption/photodegradation of amoxicillin in aqueous solutions by an integrated photocatalytic adsorbent (IPCA): Experimental studies and kinetics analysis. Photochem. Photobiol. Sci. 2011, 10, 1014–1022. [Google Scholar] [CrossRef]
- Kozlova, A.; Kirik, S.D. Post-synthetic activation of silanol covering in the mesostructured silicate, materials MCM-41 and SBA-15. Microporous Mesoporous Mater. 2010, 133, 124–133. [Google Scholar] [CrossRef]
- Kumar, D.; Schumacher, K.; du Fresne von Hohenesche, C.; Grun, M.; Unger, K.K. MCM-41, MCM-48 and related mesoporous adsorbents: Their synthesis and characterization. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187–188, 109–116. [Google Scholar] [CrossRef]
- Kishor, R.; Singh, S.B.; Ghoshal, A.K. Role of metal type on mesoporous KIT-6 for hydrogen storage. Int. J. Hydrogen Energy 2018, 43, 10376–10385. [Google Scholar] [CrossRef]
- Duan, Y.; Zhai, D.; Zhang, X.; Zheng, J.; Li, C. Synthesis of CuO/Ti-MCM-48 Photocatalyst for the Degradation of Organic Pollutions Under Solar-Simulated Irradiation. Catal. Lett. 2017, 148, 51–61. [Google Scholar] [CrossRef]
- Purushothaman, R.; Palanichamy, M.; Bilal, I.M. Functionalized KIT-6/Terpolyimide Composites with Ultra-Low Dielectric Constant. J. Appl. Polym. Sci. 2014, 131, 40508. [Google Scholar] [CrossRef]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Poell, C.J. NIST X-ray Photoelectron Spectroscopy Database; Version 4.1; NIST Standard Reference Database NIST SRD 20, National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Moulder, F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy, ULVAC-PHI, Inc, 370 Enzo, Chigasaki 253-8522, Japan; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1995. [Google Scholar]
- Zhang, Y.; Liu, J.X.; Qian, K.; Jia, A.; Li, D.; Shi, L.; Hu, J.; Zhu, J.; Huang, X. Structure Sensitivity of Au-TiO2 Strong Metal–Support Interactions. Angew. Chem. 2021, 60, 12074–12081. [Google Scholar] [CrossRef] [PubMed]
- Perera, A.S.; Trogadas, P.; Nigra, M.M.; Yu, H.; Coppens, M.O. Optimization of mesoporous titanosilicate catalysts for cyclohexene epoxidation via statistically guided synthesis. J. Mater. Sci. 2018, 53, 7279–7293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tang, C.; Lv, Y.; Gao, F.; Dong, L. Direct synthesis of Ti-SBA-15 in the self-generated acidic environment and its photodegradation of Rhodamine. J. Porous Mater. 2014, 21, 63–70. [Google Scholar] [CrossRef]
- Montagna, M. Characterization of Sol–Gel Materials by Raman and Brillouin Spectroscopies. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–32. [Google Scholar]
- Sauvajol, J.; Pelous, J.; Woignier, T.; Vacher, R. Low frequency Raman study of the harmonic vibrational modes in silica aerogels. J. Phys. Colloq. 1989, 50, 167–169. [Google Scholar] [CrossRef]
- Machon, D.; Bois, L.; Fandio, D.J.J.; Martinet, Q.; Forestier, A.; LeFloch, S.; Margueritat, J.; Pischedda, V.; Morris, D.; Saviot, L. Revisiting Pressure-induced Transitions in Mesoporous Anatase TiO2. J. Phys. Chem. C 2019, 123, 23488–23496. [Google Scholar] [CrossRef]
- Ivanda, M.; Musić, S.; Gotić, M.; Turković, A.; Tonejc, A.M.; Gamulin, O. XRD, Raman and FT-IR spectroscopic observations of nanosized TiO2 synthesized by the sol–gel method based on an esterification reaction. J. Mol. Struct. 1999, 480, 645–649. [Google Scholar] [CrossRef]
- Zhu, K.-R.; Zhang, M.S.; Chen, Q.; Yin, Z. Size and phonon-confinement effects on low-frequency Raman mode of anatase TiO2 nanocrystal. Phys. Lett. A 2005, 340, 220–227. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhang, L.; Fu, H.; He, P.; Han, D.; Lawson, T.; An, X. The Use of Tunable Optical Absorption Plasmonic Au and Ag Decorated TiO2 Structures as Efficient Visible Light Photocatalysts. Catalysts 2020, 10, 139. [Google Scholar] [CrossRef]
- Georgescu, D.; Baia, L.; Ersen, O.; Baia, M.; Simon, S. Experimental assessment of the phonon confinement in TiO2 anatase nanocrystallites by Raman spectroscopy. J. Raman Spectrosc. 2012, 43, 876–883. [Google Scholar] [CrossRef]
- Wang, T.; Luo, S.; Tompsett, G.A.; Timko, M.T.; Fan, W.; Auerbach, S.M. Critical Role of Tricyclic Bridges Including Neighboring Rings for Understanding Raman Spectra of Zeolites. J. Am. Chem. Soc. 2019, 141, 20318–20324. [Google Scholar] [CrossRef] [PubMed]
- Luan, Z.; Maes, E.M.; van der Heide, P.A.W.; Zhao, D.; Czernuszewicz, R.S.; Kevan, L. Incorporation of Titanium into Mesoporous Silica Molecular Sieve SBA-15. Chem. Mater. 1999, 11, 3680–3686. [Google Scholar] [CrossRef]
- Aguiar, H.; Serra, J.; González, P.; León, B. Structural study of sol–gel silicate glasses by IR and Raman spectroscopies. J. Non-Cryst. Solids 2009, 355, 475–480. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Zhao, Z.; Gao, M.; Kong, L.; Liu, J.; Wei, Y. Design, synthesis and catalytic performance of vanadium-incorporated mesoporous silica KIT-6 catalysts for the oxidative dehydrogenation of propane to propylene. Catal. Sci. Technol. 2016, 6, 5927–5941. [Google Scholar] [CrossRef]
- Fan, F.; Feng, Z.; Li, C. UV Raman Spectroscopic Studies on Active Sites and Synthesis Mechanisms of Transition Metal-Containing Microporous and Mesoporous Materials. Acc. Chem. Res. 2010, 43, 378–387. [Google Scholar] [CrossRef]
- Ricchiardi, G.; Damin, A.; Bordiga, S.; Lamberti, C.; Spano, G.; Rivetti, F.; Zecchina, A. Vibrational Structure of Titanium Silicate Catalysts. A Spectroscopic and Theoretical Study. J. Am. Chem. Soc. 2001, 123, 11409–11419. [Google Scholar] [CrossRef]
- Parvulescu, V.; Ciobanu, M.; Petcu, G. Immobilization of semiconductor photocatalysts. In Handbook of Smart Photocatalytic Materials Fundamentals, Fabrications, and Water Resources Applications; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Zhu, Q.; Lu, J.; Wang, Y.; Qin, F.; Shi, Z.; Xu, C. Burstein-Moss Effect Behind Au Surface Plasmon Enhanced Intrinsic Emission of ZnO Microdisks. Sci. Rep. 2016, 6, 36194. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Kaczmarek, D.; Domaradzki, J.; Mazur, M. Correlation of Photocatalysis and Photoluminescence Effect in Relation to the Surface Properties of TiO2:Tb Thin Films. Int. J. Photoenergy 2013, 2013, 526140. [Google Scholar] [CrossRef]
- Liqiang, J.; Yichun, Q.; Baiqi, W.; Shudan, L.; Baojiang, J.; Libin, Y.; Wei, F.; Honggang, F.; Jiazhong, S. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- De Lourdes Ruiz Peralta, M.; Pal, U.; Sanchez Zeferino, R. Photoluminescence (PL) Quenching and Enhanced Photocatalytic Activity of Au-Decorated ZnO Nanorods Fabricated through Microwave-Assisted Chemical Synthesis. ACS Appl. Mater. Interfaces 2012, 4, 4807–4816. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Kockler, J.; Motti, C.A.; Glass, B.D.; Oelgemoller, M. Titanium dioxide/zeolite integrated photocatalytic adsorbents for the degradation of amoxicillin. Appl. Catal. B Environ. 2014, 166–167, 45–55. [Google Scholar] [CrossRef]
- Nairi, V.; Medda, L.; Monduzzi, M.; Salis, A. Adsorption and release of ampicillin antibiotic from ordered mesoporous silica. J. Colloid Interface Sci. 2017, 497, 217–225. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.N.R.; Insa, S.; Mozeto, A.A.; Petrovic, M.; Chaves, T.F.; Fadini, P.S. Equilibrium and kinetic studies of the adsorption of antibiotics from aqueous solutions onto powdered zeolites. Chemosphere 2018, 205, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Kuzniatsova, T.; Kim, Y.; Shqau, K.; Dutta, P.K.; Verweij, H. Zeta potential measurements of zeolite Y: Application in homogeneous deposition of particle coatings. Micropor. Mesopor. Mat. 2007, 103, 102–107. [Google Scholar] [CrossRef]
- Chinh, V.D.; Broggi, A.; Di Palma, L.; Scarsella, M.; Speranza, G.; Vilardi, G.; Thang, P.N. XPS Spectra Analysis of Ti2+, Ti3+ Ions and Dye Photodegradation Evaluation of Titania-Silica Mixed Oxide Nanoparticles. J. Electron. Mater. 2018, 47, 2215–2224. [Google Scholar] [CrossRef]
- Liu, W.; He, T.; Wang, Y.; Ning, G.; Xu, Z.; Chen, X.; Hu, X.; Wu, Y.; Zhao, Y. Synergistic adsorption-photocatalytic degradation efect and norfoxacin mechanism of ZnO/ZnS@BC under UV-light irradiation. Sci. Rep. 2020, 10, 11903. [Google Scholar] [CrossRef]
- Emara, M.M.; Ali, S.H.; Hassan, A.A.; Kassem, T.S.E.; Van Patten, P.G. How does photocatalytic activity depend on adsorption, composition, and other key factors in mixed metal oxide nanocomposites. Colloid Interfac. Sci. 2021, 40, 100341. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Q.; Han, C.; Yang, Y.; Jiang, W.; Zhang, Z. Photocatalytic degradation of recalcitrant organic pollutants in water using a novel cylindrical multi-column photoreactor packed with TiO2-coated silica gel beads. J. Hazard. Mater. 2015, 285, 398–408. [Google Scholar] [CrossRef]
- Tsukamoto, D.; Shiraishi, Y.; Sugano, Y.; Ichikawa, S.; Tanaka, S.; Hirai, T. Gold Nanoparticles Located at the Interface of Anatase/Rutile TiO2 Particles as Active Plasmonic Photocatalysts for Aerobic Oxidation. J. Am. Chem. Soc. 2012, 134, 6309–6315. [Google Scholar] [CrossRef]
- Siah, W.R.; Lintang, H.O.; Shamsuddin, M.; Yuliati, L. High photocatalytic activity of mixed anatase-rutile phases on commercial TiO2 nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012005. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Chang, I.G.; Tai, Y.; Wu, K.W. Effect of surface plasmon resonance on the photocatalytic activity of Au/TiO2 under UV/visible illumination. J. Nanosci. Nanotechnol. 2012, 12, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Ye, M.; Rao, Z.; Tian, T.; Shu, L.; Lin, P.; Zeng, X.; Ke, S. Flexible TiO2/Au thin films with greatly enhanced photocurrents for photoelectrochemical water splitting. J. Alloys Compd. 2020, 815, 152471. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Zhou, D.; Xiong, H.; Zhou, Y.; Dong, S.; Rittmann, B.E. Eliminating partial-transformation products and mitigating residual toxicity of amoxicillin through intimately coupled photocatalysis and biodegradation. Chemosphere 2019, 237, 124491. [Google Scholar] [CrossRef] [PubMed]
- Elmolla, E.S.; Chaudhuri, M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination 2010, 252, 46–52. [Google Scholar] [CrossRef]
- Gozlan, I.; Rotstein, A.; Avisar, D. Amoxicillin-degradation products formed under controlled environmental conditions: Identification and determination in the aquatic environment. Chemosphere 2013, 91, 985–992. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Magnetic fluorinated mesoporous g-C3N4 for photocatalytic degradation of amoxicillin: Transformation mechanism and toxicity assessment. Appl. Catal. B Environ. 2019, 242, 337–348. [Google Scholar] [CrossRef]











| Sample | Binding Energy (eV) | Au Chemical Species rel.conc. | ||||||
|---|---|---|---|---|---|---|---|---|
| Au4f/2 Metallic nps | Au4f/2 Clusters | Au4f/2 Au+ | Au4f/2 Au3+ | Au Metallic nps | Clusters | Au1+ | Au3+ | |
| YTA | 83.3 | 84.3 | 85.3 | 86.7 | 44.7 | 18.8 | 18 | 18.6 |
| hYTA | 83.3 | 84.3 | 85.3 | 86.7 | - | 60.2 | - | 39.8 |
| MTA | 83.3 | 84.3 | 85.3 | 86.7 | - | 63.9 | - | 36.1 |
| KTA | 83.3 | 84.3 | 85.3 | 86.7 | 64.8 | - | - | 35.2 |
| Sample | YT | hYT | KT | MT |
| Eg (eV) | 3.17 | 3.22 | 3.14 | 3.13 |
| Sample | YTA | hYTA | KTA | MTA |
| Eg (eV) | 3.20 | 3.26 | 3.19 | 3.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petcu, G.; Anghel, E.M.; Buixaderas, E.; Atkinson, I.; Somacescu, S.; Baran, A.; Culita, D.C.; Trica, B.; Bradu, C.; Ciobanu, M.; et al. Au/Ti Synergistically Modified Supports Based on SiO2 with Different Pore Geometries and Architectures. Catalysts 2022, 12, 1129. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12101129
Petcu G, Anghel EM, Buixaderas E, Atkinson I, Somacescu S, Baran A, Culita DC, Trica B, Bradu C, Ciobanu M, et al. Au/Ti Synergistically Modified Supports Based on SiO2 with Different Pore Geometries and Architectures. Catalysts. 2022; 12(10):1129. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12101129
Chicago/Turabian StylePetcu, Gabriela, Elena Maria Anghel, Elena Buixaderas, Irina Atkinson, Simona Somacescu, Adriana Baran, Daniela Cristina Culita, Bogdan Trica, Corina Bradu, Madalina Ciobanu, and et al. 2022. "Au/Ti Synergistically Modified Supports Based on SiO2 with Different Pore Geometries and Architectures" Catalysts 12, no. 10: 1129. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12101129