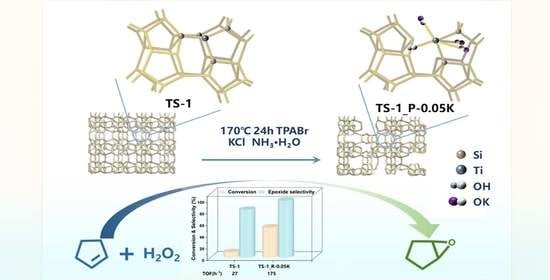
Hydrothermal Modification of TS-1 Zeolites with Organic Amines and Salts to Construct Highly Selective Catalysts for Cyclopentene Epoxidation
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Characterization
2.2. Catalytic Tests
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis
4.2.1. Synthesis of the Parent TS-1 Zeolite
4.2.2. Hydrothermal Modification
4.3. Characterizations
4.4. Catalytic Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, Z.Q.; Bian, W.; Liu, L.S.; Lu, Z. Catalytic oxidation of cyclopentene to glutaraldehyde over WO3/Ti-HMS catalyst. Catal. Lett. 2007, 117, 79–84. [Google Scholar] [CrossRef]
- Xue, J.J.; Wang, A.L.; Yin, H.B.; Wang, J.B.; Zhang, D.Z.; Chen, W.G.; Yu, L.B.; Jiang, T.S. Oxidation of cyclopentene catalyzed by phosphotungstic quaternary ammonium salt catalysts. J. Ind. Eng. Chem. 2010, 16, 288–292. [Google Scholar] [CrossRef]
- Zhang, W.; Du, B. The OH-initiated atmospheric oxidation of cyclopentene: A coupled-cluster study of the potential energy surface. Chem. Phys. Lett. 2013, 579, 35–39. [Google Scholar] [CrossRef]
- Kluson, P.; Luskova, H.; Cerveny, L.; Klisakova, J.; Cajthaml, T. Partial photocatalytic oxidation of cyclopentene over titanium(IV) oxide. J. Mol. Catal. A-Chem. 2005, 242, 62–67. [Google Scholar] [CrossRef]
- Tong, W.; Yin, J.; Ding, L.; Xu, H.; Wu, P. Modified Ti-MWW Zeolite as a Highly Efficient Catalyst for the Cyclopentene Epoxidation Reaction. Front. Chem. 2020, 8, 585347. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, C.; Yue, H.; Tang, S.; Zhu, Y.; Liang, B. Selective oxidation of cyclopentene with H2O2 by using H3PW12O40 and TBAB as a phase transfer catalyst. Chin. J. Chem. Eng. 2019, 27, 1851–1856. [Google Scholar] [CrossRef]
- Mel’nik, L.V.; Meshechkina, A.E.; Rybina, G.V.; Srednev, S.S.; Moskvichev, Y.A.; Kozlova, O.S. Synthesis of 1,2-epoxycyclopentane and/or 1,2-cyclopentanediol by oxidation of cyclopentene with aqueous solution of hydrogen peroxide. Pet. Chem. 2012, 52, 313–317. [Google Scholar] [CrossRef]
- Xue, J.J.; Yin, H.B.; Li, H.X.; Zhang, D.Z.; Jiang, T.S.; Yu, L.B.; Shen, Y.T. Oxidation of cyclopentene catalyzed by tungsten-substituted molybdophosphoric acids. Korean J. Chem. Eng. 2009, 26, 654–659. [Google Scholar] [CrossRef]
- Chung, W.C.; Darensbourg, D.J. Copolymerization of cyclopentene oxide with CO2 utilizing bifunctional cobalt(III)- and chromium(III)-salen catalysts. Abstr. Am. Chem. Soc. 2014, 247, 771-INOR. [Google Scholar]
- Darensbourg, D.J.; Chung, W.C.; Wilson, S.J. Catalytic Coupling of Cyclopentene Oxide and CO2 Utilizing Bifunctional (salen)Co(III) and (salen)Cr(III) Catalysts: Comparative Processes Involving Binary (salen)Cr(III) Analogs. ACS Catal. 2013, 3, 3050–3057. [Google Scholar] [CrossRef]
- Pramanik, A.; Abbina, S.; Das, G. Molecular, supramolecular structure and catalytic activity of transition metal complexes of phenoxy acetic acid derivatives. Polyhedron 2007, 26, 5225–5234. [Google Scholar] [CrossRef]
- Pałasz, A. Three-component one-pot synthesis of fused uracils – pyrano[2,3-d]-pyrimidine-2,4-diones. Mon. Chem. 2008, 139, 1397. [Google Scholar] [CrossRef]
- Maiti, S.K.; Dinda, S.; Bhattacharyya, R. Unmatched efficiency and selectivity in the epoxidation of olefins with oxo-diperoxomolybdenum(VI) complexes as catalysts and hydrogen peroxide as terminal oxidant. Tetrahedron Lett. 2008, 49, 6205–6208. [Google Scholar] [CrossRef]
- Qi, J.-Y.; Li, Y.-M.; Zhou, Z.-Y.; Che, C.-M.; Yeung, C.-H.; Chan, A.S.C. Novel Manganese Complex as an Efficient Catalyst for the Isobutyraldehyde-Mediated Epoxidation of Cyclic Alkenes with Dioxygen. Adv. Synth. Catal. 2005, 347, 45–49. [Google Scholar] [CrossRef]
- Mekrattanachai, P.; Liu, J.; Li, Z.H.; Cao, C.Y.; Song, W.G. Extremely low loading of Ru species on hydroxyapatite as an effective heterogeneous catalyst for olefin epoxidation. Chem. Commun. 2018, 54, 1433–1436. [Google Scholar] [CrossRef]
- Li, S.; Shi, L.; Zhang, L.; Huang, H.; Xiao, Y.; Mao, L.; Tan, R.; Fu, Z.; Yu, N.; Yin, D. Ionic liquid-mediated catalytic oxidation of β-caryophyllene by ultrathin 2D metal-organic framework nanosheets under 1 atm O2. Mol. Catal. 2020, 496, 111196. [Google Scholar] [CrossRef]
- Ren, J.; Wang, L.; Li, P.; Xing, X.; Wang, H.; Lv, B. Ag supported on alumina for the epoxidation of 1-hexene with molecular oxygen: The effect of Ag+/Ag0. New J. Chem. 2022, 46, 4792–4799. [Google Scholar] [CrossRef]
- Wang, F.; Meng, X.-G.; Wu, Y.-Y.; Huang, H.; Lv, J.; Yu, W.-W. A Highly Efficient Heterogeneous Catalyst of Bimetal-Organic Frameworks for the Epoxidation of Olefin with H2O2. Molecules 2020, 25, 2389. [Google Scholar] [CrossRef]
- Jin, M.M.; Guo, Z.M.; Lv, Z.G. Immobilization of tungsten chelate complexes on functionalized mesoporous silica SBA-15 as heterogeneous catalysts for oxidation of cyclopentene. J. Mater. Sci. 2019, 54, 6853–6866. [Google Scholar] [CrossRef]
- Hulea, V.; Dumitriu, E.; Patcas, F.; Ropot, R.; Graffin, P.; Moreau, P. Cyclopentene oxidation with H2O2 over Ti-containing zeolites. Appl. Catal. A-Gen. 1998, 170, 169–175. [Google Scholar] [CrossRef]
- Wu, P.; Nuntasri, D.; Liu, Y.M.; Wu, H.H.; Jiang, Y.W.; Fan, W.B.; He, M.Y.; Tatsumi, T. Selective liquid-phase oxidation of cyclopentene over MWW type titanosilicate. Catal. Today 2006, 117, 199–205. [Google Scholar] [CrossRef]
- Hincapie, B.; Llano, S.M.; Garces, H.F.; Espinal, D.; Suib, S.L.; Garces, L.J. Epoxidation of cyclopentene by a low cost and environmentally friendly bicarbonate/peroxide/manganese system. Adsorpt. Sci. Technol. 2018, 36, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Sujandi; Han, S.-C.; Han, D.-S.; Jin, M.-J.; Park, S.-E. Catalytic oxidation of cycloolefins over Co(cyclam)-functionalized SBA-15 material with H2O2. J. Catal. 2006, 243, 410–419. [Google Scholar] [CrossRef]
- Niu, Q.T.; Liu, G.D.; Lv, Z.G.; Si, C.D.; Jin, M.M. Assembly of SBA-15 Derived Hybrid Heterogeneous Catalysts for Liquid Phase Cyclopentene Epoxidation. ChemistrySelect 2021, 6, 2111–2118. [Google Scholar] [CrossRef]
- Tong, J.H.; Liu, F.F.; Wang, W.H.; Bo, L.L.; Mahboob, A.; Fan, H.Y. Highly Efficient Epoxidation of Cyclopentene Catalyzed by Magnetically Recoverable Mg-doped Cobalt Ferrites with Greatly Improved Performances. ChemistrySelect 2016, 1, 6356–6361. [Google Scholar] [CrossRef]
- Cui, X.Z.; Shi, J.L. Sn-based catalysts for Baeyer-Villiger oxidations by using hydrogen peroxide as oxidant. Sci. China-Mater. 2016, 59, 675–700. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, W.; Rahman, A.U.; Ahmad, I.; Yaseen, M.; Jan, B.M.; Stylianakis, M.M.; Kenanakis, G.; Ikram, R. Oxidative Desulfurization of Petroleum Distillate Fractions Using Manganese Dioxide Supported on Magnetic Reduced Graphene Oxide as Catalyst. Nanomaterials 2021, 11, 203. [Google Scholar] [CrossRef]
- Song, X.J.; Yang, X.T.; Zhang, T.J.; Zhang, H.; Zhang, Q.; Hu, D.W.; Chang, X.Y.; Li, Y.Y.; Chen, Z.Y.; Jia, M.J.; et al. Controlling the Morphology and Titanium Coordination States of TS-1 Zeolites by Crystal Growth Modifier. Inorg. Chem. 2020, 59, 13201–13210. [Google Scholar] [CrossRef]
- Wang, H.; Du, G.; Chen, S.; Su, Z.; Sun, P.; Chen, T. Steam-assisted strategy to fabricate Anatase-free hierarchical titanium Silicalite-1 Single-Crystal for oxidative desulfurization. J. Colloid Interface Sci. 2022, 617, 32–43. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, L.L.; Yu, Y.K.; Liu, D.X.; Fang, N.; Lin, Y.X.; Xu, D.Y.; Li, F.; Liu, Y.M.; He, M.Y. TS-1 zeolite with homogeneous distribution of Ti atoms in the framework: Synthesis, crystallization mechanism and its catalytic performance. J. Catal. 2021, 404, 990–998. [Google Scholar] [CrossRef]
- Smeets, V.; Gaigneaux, E.M.; Debecker, D.P. Titanosilicate Epoxidation Catalysts: A Review of Challenges and Opportunities. ChemCatChem 2022, 14, 1–25. [Google Scholar] [CrossRef]
- Yang, G.J.; Han, J.; Liu, Y.; Qiu, Z.Y.; Chen, X.X. The synthetic strategies of hierarchical TS-1 zeolites for the oxidative desulfurization reactions. Chin. J. Chem. Eng. 2020, 28, 2227–2234. [Google Scholar] [CrossRef]
- Zhou, W.; Meng, F.L.; Xu, Q.H.; Dong, J.L.; Chun, Y. Preparation of Amphiphilic TS-1 zeolites and their phase-boundary catalysis. Acta Chim. Sin. 2004, 62, 1425–1429. [Google Scholar]
- Kong, L.; Li, G.; Wang, X. Mild oxidation of thiophene over TS-1/H2O2. Catal. Today 2004, 93-95, 341–345. [Google Scholar] [CrossRef]
- Liu, N.; Guo, H.; Wang, X.; Chen, L.; Zoub, L. Increasing the propylene epoxidation activity of TS-1 catalysts by hydrothermal treatment of ammonia solution. React. Kinet. Catal. Lett. 2005, 87, 77–83. [Google Scholar] [CrossRef]
- Liu, H.; Lu, G.; Guo, Y.; Guo, Y.; Wang, J. Effect of pretreatment on properties of TS-1/diatomite catalyst for hydroxylation of phenol by H2O2 in fixed-bed reactor. Catal. Today 2004, 93-95, 353–357. [Google Scholar] [CrossRef]
- Yu, Y.K.; Tang, Z.M.; Liu, W.; Wang, J.; Chen, Z.; Shen, K.X.; Wang, R.; Liu, H.X.; Huang, X.; Liu, Y.M. Enhanced catalytic oxidation performance of K+-modified Ti-MWW through selective breaking of interfacial hydrogen-bonding interactions of H2O2. Appl. Catal. A-Gen. 2019, 587, 117270. [Google Scholar] [CrossRef]
- Wang, Y.R.; Lin, M.; Tuel, A. Hollow TS-1 crystals formed via a dissolution-recrystallization process. Microporous Mesoporous Mat. 2007, 102, 80–85. [Google Scholar] [CrossRef]
- Tatsumi, T.; Koyano, K.A.; Shimizu, Y. Effect of potassium on the catalytic activity of TS-1. Appl. Catal. A-Gen 2000, 200, 125–134. [Google Scholar] [CrossRef]
- Du, S.T.; Li, F.; Sun, Q.M.; Wang, N.; Jia, M.J.; Yu, J.H. A green surfactant-assisted synthesis of hierarchical TS-1 zeolites with excellent catalytic properties for oxidative desulfurization. Chem. Commun. 2016, 52, 3368–3371. [Google Scholar] [CrossRef]
- Du, S.T.; Sun, Q.M.; Wang, N.; Chen, X.X.; Jia, M.J.; Yu, J.H. Synthesis of hierarchical TS-1 zeolites with abundant and uniform intracrystalline mesopores and their highly efficient catalytic performance for oxidation desulfurization. J. Mater. Chem. A 2017, 5, 7992–7998. [Google Scholar] [CrossRef]
- Chang, X.Y.; Yang, X.T.; Song, X.J.; Xu, L.F.; Hu, D.W.; Sun, Y.T.; Jia, M.J. Addition of polyethylene glycol for the synthesis of anatase-free TS-1 zeolites with excellent catalytic activity in 1-hexene epoxidation. J. Porous Mat. 2022, 29, 641–649. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Yang, X.T.; Song, X.J.; Chang, X.Y.; Jia, M.J. Hydrothermal synthesis of tungsten-tin bimetallic MFI type zeolites and their catalytic properties for cyclohexene epoxidation. Microporous Mesoporous Mat. 2020, 303, 110277. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Xu, L.F.; Chang, X.Y.; Miao, S.S.; Sun, Y.T.; Jia, M.J. Direct hydrothermal synthesis of Mo-containing MFI zeolites using Mo-EDTA complex and their catalytic application in cyclohexene epoxidation. Chin. J. Catal. 2021, 42, 2265–2274. [Google Scholar] [CrossRef]
- Olson, D.H.; Kokotailo, G.T.; Lawton, S.L.; Meier, W.M. Crystal structure and structure-related properties of ZSM-5. J. Phys. Chem. 1981, 85, 2238–2243. [Google Scholar] [CrossRef]
- Gao, X.; An, J.G.; Gu, J.L.; Li, L.; Li, Y.S. A green template-assisted synthesis of hierarchical TS-1 with excellent catalytic activity and recyclability for the oxidation of 2,3,6-trimethylphenol. Microporous Mesoporous Mater. 2017, 239, 381–389. [Google Scholar] [CrossRef]
- Liu, M.; Chang, Z.H.; Wei, H.J.; Li, B.J.; Wang, X.Y.; Wen, Y.Q. Low-cost synthesis of size-controlled TS-1 by using suspended seeds: From screening to scale-up. Appl. Catal. A-Gen. 2016, 525, 59–67. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Yan, H.; Chen, Y.; Su, Q. Effect of sodium ions on propylene epoxidation catalyzed by titanium silicalite. Appl. Catal. A-Gen. 2001, 218, 31–38. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, X.; Chen, G.; Chen, M.; Bai, R.; Jia, M.; Yu, J. Synthesis of anatase-free nano-sized hierarchical TS-1 zeolites and their excellent catalytic performance in alkene epoxidation. J. Mater. Chem. A 2018, 6, 9473–9479. [Google Scholar] [CrossRef]
- Choi, M.; Srivastava, R.; Ryoo, R. Organosilane surfactant-directed synthesis of mesoporous aluminophosphates constructed with crystalline microporous frameworks. Chem. Commun. 2006, 42, 4380–4382. [Google Scholar] [CrossRef]
- Xi, D.Y.; Sun, Q.M.; Chen, X.X.; Wang, N.; Yu, J.H. The recyclable synthesis of hierarchical zeolite SAPO-34 with excellent MTO catalytic performance. Chem. Commun. 2015, 51, 11987–11989. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.R.; Peng, X.X.; Zhang, W.F.; Lin, M.; Zhu, B.; Liao, W.L.; Guo, X.H.; Shu, X.T. Hierarchical TS-1 synthesized via the dissolution-recrystallization process: Influence of ammonium salts. Catal. Commun. 2017, 101, 26–30. [Google Scholar] [CrossRef]
- Liu, C.; Huang, J.L.; Sun, D.H.; Zhou, Y.; Jing, X.L.; Du, M.M.; Wang, H.T.; Li, Q.B. Anatase type extra-framework titanium in TS-1: A vital factor influencing the catalytic activity toward styrene epoxidation. Appl. Catal. A-Gen. 2013, 459, 1–7. [Google Scholar] [CrossRef]
- Tsunoji, N.; Nishida, H.; Ide, Y.; Komaguchi, K.; Hayakawa, S.; Yagenji, Y.; Sadakane, M.; Sano, T. Photocatalytic Activation of C-H Bonds by Spatially Controlled Chlorine and Titanium on the Silicate Layer. ACS Catal. 2019, 9, 5742–5751. [Google Scholar] [CrossRef]
- Sasaki, M.; Sato, Y.; Tsuboi, Y.; Inagaki, S.; Kubota, Y. Ti-YNU-2: A Microporous Titanosilicate with Enhanced Catalytic Performance for Phenol Oxidation. ACS Catal. 2014, 4, 2653–2657. [Google Scholar] [CrossRef]
- Li, C.; Xiong, G.; Xin, Q.; Liu, J.K.; Ying, P.L.; Feng, Z.C.; Li, J.; Yang, W.B.; Wang, Y.Z.; Wang, G.R.; et al. UV resonance Raman spectroscopic identification of titanium atoms in the framework of TS-1 zeolite. Angew. Chem.-Int. Edit. 1999, 38, 2220–2222. [Google Scholar] [CrossRef]
- Li, C.; Xiong, G.; Liu, J.K.; Ying, P.L.; Xin, Q.; Feng, Z.C. Identifying framework titanium in TS-1 zeolite by UV resonance Raman spectroscopy. J. Phys. Chem. B 2001, 105, 2993–2997. [Google Scholar] [CrossRef]
- Guo, Q.; Feng, Z.C.; Li, G.N.; Fan, F.T.; Li, C. Finding the "Missing Components" during the Synthesis of TS-1 Zeolite by UV Resonance Raman Spectroscopy. J. Phys. Chem. C 2013, 117, 2844–2848. [Google Scholar] [CrossRef]
- Fan, F.T.; Xu, Q.; Xia, H.A.; Sun, K.J.; Feng, Z.C.; Li, C. UV Raman Spectroscopic Characterization of Catalytic Materials. Chin. J. Catal. 2009, 30, 717–739. [Google Scholar]
- Wu, L.; Zhao, S.; Lin, L.; Fang, X.; Liu, Y.; He, M. In-depth understanding of acid catalysis of solvolysis of propene oxide over titanosilicates and titanosilicate/H2O2 systems. J. Catal. 2016, 337, 248–259. [Google Scholar] [CrossRef]
- Morrow, B.A.; McFarlan, A.J. Surface vibrational modes of silanol groups on silica. J. Phys. Chem. 1992, 96, 1395–1400. [Google Scholar] [CrossRef]
- Zecchina, A.; Bordiga, S.; Spoto, G.; Marchese, L.; Petrini, G.; Leofanti, G.; Padovan, M. Silicalite characterization. 2. IR spectroscopy of the interaction of carbon monoxide with internal and external hydroxyl groups. J. Phys. Chem. 1992, 96, 4991–4997. [Google Scholar] [CrossRef]
- Halasz, I.; Agarwal, M.; Senderov, E.; Marcus, B. Continuous monitoring the oxyfunctionalization of hexane by aqueous H2O2 over TS-1 related catalysts. Appl. Catal. A-Gen 2003, 241, 167–184. [Google Scholar] [CrossRef]
- Astorino, E.; Peri, J.B.; Willey, R.J.; Busca, G. Spectroscopic Characterization of Silicalite-1 and Titanium Silicalite-1. J. Catal. 1995, 157, 482–500. [Google Scholar] [CrossRef]
- De Man, A.J.M.; Sauer, J. Coordination, Structure, and Vibrational Spectra of Titanium in Silicates and Zeolites in Comparison with Related Molecules. An ab Initio Study. J. Phys. Chem. 1996, 100, 5025–5034. [Google Scholar] [CrossRef]
- Rodenas, Y.; Fierro, J.L.G.; Mariscal, R.; Retuerto, M.; López Granados, M. Post-synthesis Treatment of TS-1 with TPAOH: Effect of Hydrophobicity on the Liquid-Phase Oxidation of Furfural to Maleic Acid. Top. Catal. 2019, 62, 560–569. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, K.; Feng, Z.; Li, G.; Guo, M.; Fan, F.; Li, C. A Thorough Investigation of the Active Titanium Species in TS-1 Zeolite by In Situ UV Resonance Raman Spectroscopy. Chem.-Eur. J. 2012, 18, 13854–13860. [Google Scholar] [CrossRef] [PubMed]
- Langhendries, G.; De Vos, D.E.; Baron, G.V.; Jacobs, P.A. Quantitative sorption experiments on Ti-zeolites and relation with alpha-olefin oxidation by H2O2. J. Catal. 1999, 187, 453–463. [Google Scholar] [CrossRef]
- Su, J.; Xiong, G.; Zhou, J.; Liu, W.; Zhou, D.; Wang, G.; Wang, X.; Guo, H. Amorphous Ti species in titanium silicalite-1: Structural features, chemical properties, and inactivation with sulfosalt. J. Catal. 2012, 288, 1–7. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Nishizawa, K.; Nakajima, T.; Kamegawa, T.; Mori, K.; Yamashita, H. Enhanced Catalytic Activity on Titanosilicate Molecular Sieves Controlled by Cation−π Interactions. J. Am. Chem. Soc. 2011, 133, 12462–12465. [Google Scholar] [CrossRef]






| Sample | RC a (%) | Si/Ti b | Si/K b | SBET c (m2/g) | Smicro c (m2/g) | Sext d (m2/g) | Vmicro d (cm3/g) | Vmeso e (cm3/g) |
|---|---|---|---|---|---|---|---|---|
| TS-1 | 100 | 56.1 | / | 399 | 285 | 114 | 0.12 | 0.02 |
| TS-1_P | 49 | 53.6 | / | 320 | 110 | 210 | 0.07 | 0.20 |
| TS-1_0.05K | 74 | 54.0 | 177 | 313 | 105 | 208 | 0.07 | 0.19 |
| TS-1_P-0.01K | 58 | 46.7 | 312 | 309 | 92 | 217 | 0.06 | 0.19 |
| TS-1_P-0.05K | 46 | 54.8 | 124 | 415 | 172 | 243 | 0.10 | 0.26 |
| TS-1_P-0.10K | 49 | 48.1 | 135 | 402 | 145 | 257 | 0.09 | 0.23 |
| Catalyst | Conv. (%) | Sel. (%) | TOF b (h−1) |
|---|---|---|---|
| TS-1 | 9.6 | 82.3 | 27 |
| TS-1_P | 13.0 | 39.8 | 17 |
| TS-1_0.05K | 28.8 | 98.8 | 94 |
| TS-1_P-0.01K | 16.4 | 90.0 | 42 |
| TS-1_P-0.05K | 52.0 | 98.2 | 175 |
| TS-1_P-0.10K | 26.7 | 98.9 | 78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, X.-Y.; Sun, Y.-T.; Song, X.-J.; Yang, X.-T.; Wu, Y.-Q.; Jia, M.-J. Hydrothermal Modification of TS-1 Zeolites with Organic Amines and Salts to Construct Highly Selective Catalysts for Cyclopentene Epoxidation. Catalysts 2022, 12, 1241. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12101241
Chang X-Y, Sun Y-T, Song X-J, Yang X-T, Wu Y-Q, Jia M-J. Hydrothermal Modification of TS-1 Zeolites with Organic Amines and Salts to Construct Highly Selective Catalysts for Cyclopentene Epoxidation. Catalysts. 2022; 12(10):1241. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12101241
Chicago/Turabian StyleChang, Xin-Yu, Yu-Ting Sun, Xiao-Jing Song, Xiao-Tong Yang, Yu-Qing Wu, and Ming-Jun Jia. 2022. "Hydrothermal Modification of TS-1 Zeolites with Organic Amines and Salts to Construct Highly Selective Catalysts for Cyclopentene Epoxidation" Catalysts 12, no. 10: 1241. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12101241