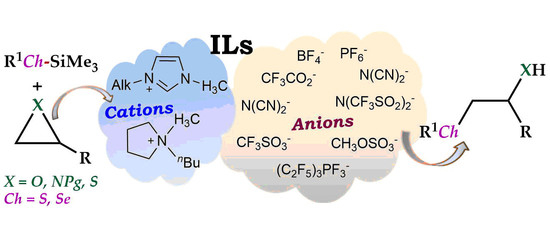
Ionic Liquids-Assisted Ring Opening of Three-Membered Heterocycles with Thio- and Seleno-Silanes
Abstract
:1. Introduction
2. Results
2.1. Reaction of Thio- and Selenosilanes with Epoxides
2.2. Reaction of Thio- and Selenosilanes with Aziridines
2.3. Reaction of Thio- and Selenosilanes with Thiiranes
3. Materials and Methods
3.1. Instruments and Reagents
3.2. Experimental Method
3.2.1. General Procedure for the Ring Opening of Epoxides 1 by (phenylthio)trimethylsilane 2a and (phenylseleno)trimethylsilane 13
3.2.2. General Procedure for the Reaction of Epoxides with bis(trimethylsilyl)sulfide 2b
3.2.3. General Procedure for the Reaction of N-Ts-Aziridine 16 with Silyl Nucleophiles 2a and 13
3.2.4. General Procedure for the Reaction of N-Boc Aziridines with (phenylthio)trimethylsilane 2a and (phenylseleno)trimethylsilane 13
3.2.5. Reaction of Aziridine 19a with bis(trimethylsilyl)sulfide 2b
3.2.6. General Procedure for the Ring Opening of Thiiranes 24
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thurakkal, L.; Singh, S.; Roy, S.; Kar, P.; Sadhukhan, S.; Porel, M. An in-silico study on selected organosulfur compounds as potential drugs for SARS-CoV-2 infection via binding multiple drug targets. Chem. Phys. Lett. 2021, 763, 138193. [Google Scholar] [CrossRef] [PubMed]
- Ruhee, R.T.; Roberts, L.A.; Ma, S.; Suzuki, K. Organosulfur Compounds: A review of their anti-inflammatory effects in human health. Front. Nutr. 2020, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, M.; Nakamura, S.; Hayashida, H.; Noguchi, D.; Nakashima, S.; Yoneda, T.; Matsuda, H. Structures of cyclic organosulfur compounds from garlic (Allium sativum L.) leaves. Front. Chem. 2020, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Block, E. Garlic and Other Alliums: The Lore and the Science; The Royal Society of Chemistry: Cambridge, MA, USA, 2009; p. 454. [Google Scholar]
- Miękus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Świergiel, A.H. Health benefits of plant-derived sulfur compounds, glucosinolates, and organosulfur compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef]
- Shadike, Z.; Tan, S.; Wang, Q.-C.; Lin, R.; Hu, E.; Qu, D.; Yang, X.-Q. Review on organosulfur materials for rechargeable lithium batteries. Mater. Horiz. 2021, 8, 471–500. [Google Scholar] [CrossRef]
- Copini, S.; Micheletti, A.C.; de Lima, D.P.; Gomes, R.S.; Mezad, A.; Beatriz, A. Synthesis and antioxidant and antimicrobial properties of β-hydroxy sulfides, sulfoxides, and sulfones derived from cardanol and glycerol derivatives. J. Braz. Chem. Soc. 2020, 31, 2569–2582. [Google Scholar] [CrossRef]
- Marakalala, M.B.; Mmutlane, E.M.; Kinfe, H.H. β-Hydroxy sulfides and their syntheses. Beilstein J. Org. Chem. 2018, 14, 1668–1692. [Google Scholar] [CrossRef]
- Azizi, B.; Poor Heravi, M.R.; Hossaini, Z.; Ebadid, A.; Vessally, E. Intermolecular difunctionalization of alkenes: Synthesis of β-hydroxy sulfides. RSC Adv. 2021, 11, 13138–13151. [Google Scholar] [CrossRef]
- Chen, Z.; Nasr, S.M.; Kazemi, M.; Mohammadi, M. A mini-review: Achievements in the thiolysis of epoxides. Mini-Rev. Org. Chem. 2020, 17, 352–362. [Google Scholar] [CrossRef]
- Guo, W.; Chen, J.; Wu, D.; Ding, J.; Chen, F.; Wu, H. Rongalite® promoted highly regioselective synthesis of β-hydroxy sulfides by ring opening of epoxides with disulfides. Tetrahedron 2009, 65, 5240–5243. [Google Scholar] [CrossRef]
- Fringuelli, F.; Pizzo, F.; Vaccaro, L. NaOH-Catalyzed Thiolysis of α,β-Epoxyketones in Water. A Key Step in the Synthesis of Target Molecules Starting from α,β-Unsaturated Ketones. J. Org. Chem. 2004, 69, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Gawronski, J.; Wascinska, N.; Gajewy, J. Recent Progress in Lewis Base Activation and Control of Stereoselectivity in the Additions of Trimethylsilyl Nucleophiles. Chem. Rev. 2008, 108, 5227–5252. [Google Scholar]
- Baker, A.; Wirth, T. Silyl Sulfides and Selenides (Updates 2017). Sci. Synth. Knowl. Updates 2017, 1, 189–202. [Google Scholar] [CrossRef]
- Capperucci, A.; Tiberi, C.; Pollicino, S.; Degl’Innocenti, A. Tetrabutylammonium phenoxide induced reaction of silyl nucleophiles. Tetrahedron Lett. 2009, 50, 2808–2810. [Google Scholar] [CrossRef]
- Degl’Innocenti, A.; Pollicino, S.; Capperucci, A. Synthesis and stereoselective functionalization of silylated heterocycles as a new class of formyl anion equivalents. Chem. Commun. 2006, 4881–4893. [Google Scholar] [CrossRef] [PubMed]
- Degl’Innocenti, A.; Capperucci, A.; Cerreti, A.; Pollicino, A.; Scapecchi, S.; Malesci, I.; Castagnoli, G. Regio- and enantioselective ring opening of epoxides with HMDST: A straighforward access to 1,2-mercaptoalcohols. Synlett 2005, 2005, 3063–3066. [Google Scholar] [CrossRef]
- Tanabe, Y.; Mori, K.; Yoshida, Y. Mild, effective and regioselective ring-opening of oxiranes usingseveral thiosilanes promoted by tetrabutylammonium fluoride as catalyst. J. Chem. Soc. Perkin Trans. 1 1997, 671–675. [Google Scholar] [CrossRef]
- Tanini, D.; Degl’Innocenti, A.; Capperucci, A. Bis(trimethylsilyl)selenide in the selective synthesis of β-hydroxy, β-mercapto, and β-amino diorganyl diselenides and selenides through ring opening of strained heterocycles. Eur. J. Org. Chem. 2015, 2015, 357–369. [Google Scholar] [CrossRef]
- Tanini, D.; Tiberi, C.; Gellini, C.; Salvi, P.R.; Capperucci, A. A straightforward access to stable β-functionalized alkyl selenols. Adv. Synth. Catal. 2018, 360, 3367–3375. [Google Scholar] [CrossRef]
- Marullo, S.; D’Anna, F.; Rizzo, C.; Billeci, F. Ionic liquids: “Normal” solvents or nanostructured fluids? Org. Biomol. Chem. 2021, 19, 2076–2095. [Google Scholar] [CrossRef]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Wasserscheid, P.; Welton, T. (Eds.) Ionic Liquids in Synthesis, 2nd ed.; Wiley: Hoboken, NJ, USA, 2008; ISBN 978-3-527-31239-9. [Google Scholar]
- Chiappe, C.; Pieraccini, D. Ionic liquids: Solvent properties and organic reactivity. J. Phys. Org. Chem. 2005, 18, 275–297. [Google Scholar] [CrossRef]
- Dupont, J.; Spencer, J. On the noninnocent nature of 1,3-dialkylimidazolium ionic liquids. Angew. Chem. Int. Ed. 2004, 43, 5296–5297. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, A.R.; Rafiee, F. Basic ionic liquids. A short review. J. Iran. Chem. Soc. 2009, 6, 647–678. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Pringle, J.M.; Johansson, K.M.; Forsyth, S.A.; Forsyth, M. Lewis base ionic liquids. Chem. Commun. 2006, 1905–1917. [Google Scholar] [CrossRef]
- Joseph, T.; Sahoo, S.; Halligudi, S.B. Brönsted acidic ionic liquids: A green, efficient and reusable catalyst system and reaction medium for Fischer esterification. J. Mol. Catal. A Chem. 2005, 234, 107–110. [Google Scholar] [CrossRef]
- Sheldon, R. Catalytic reactions in ionic liquids. Chem. Commun. 2001, 2399–2407. [Google Scholar] [CrossRef]
- Gordon, C.M. New developments in catalysis using ionic liquids. Appl. Catal. A Gen. 2001, 222, 101–117. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, G.; Li, Y.; He, B.; Liu, R.; Zhang, S. Epoxide ring-opening reaction promoted by ionic liquid reactivity: Interplay of experimental and theoretical studies. Catal. Sci. Technol. 2019, 9, 5567–5571. [Google Scholar] [CrossRef]
- Hirschberg, M.E.; Ignat´ev, N.V.; Wenda, A.; Willner, H. Selective elemental fluorination in ionic liquids. J. Fluorine Chem. 2012, 137, 50–53. [Google Scholar] [CrossRef]
- Ignat´ev, N.V.; Schulte, M.; Zlotin, S.G.; Makhova, N.N.; Sheremetev, A.B. Ionic liquids—A superior reaction media for organic syntheses with dangerous reagents. In Proceedings of the 10th German-Russian-Ukrainian Symposium on Fluorine Chemistry, Berlin, Germany, 26–28 November 2014. [Google Scholar]
- Epishina, M.A.; Kulikov, A.S.; Makhova, N.N.; Ignat´ev, N.V.; Schulte, M. The first example of the Schmidt reaction in ionic liquids. Mendeleev Comm. 2010, 20, 335–336. [Google Scholar] [CrossRef]
- Ignat´ev, N.V.; Schulte, M.; Koppe, K.; Barthen, P.; Zlotin, S.G.; Makhova, N.N.; Sheremetev, A.B.; Valente, A.A. Ionic Liquids—Advanced Reaction Media for Organic Synthesis. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 1205–1216. [Google Scholar] [CrossRef]
- Ignat´ev, N.V.; Welz-Biermann, U.; Heckmeier, M.; Oldenburg, N.; Koppe, K.; Barthen, P.; Frohn, H.-J. Dehydration of Alcohols to form Alkenes or Ethers. WO 2007/014613 29 March 2007. [Google Scholar]
- Lima, S.; Neves, P.; Antunes, M.M.; Pillinger, M.; Ignat´ev, N.V.; Valente, A.A. Conversion of mono/di/polysaccharides into furan compounds using 1-alkyl-3-methylimidazolium ionic liquids. Appl. Catal. A Gen. 2009, 363, 93–99. [Google Scholar] [CrossRef]
- Ignat´ev, N.V.; Koppe, K.; Barthen, P.; Frohn, H.-J. Synthesis of chromane derivatives in the presence of ionic liquids. WO2008086847A2, 24 July 2008. Available online: https://patents.google.com/patent/WO2008086847A2/de (accessed on 15 July 2022).
- Epishina, M.A.; Kulikov, A.S.; Ignat´ev, N.V.; Schulte, M.; Makhova, N.N. Ionic liquid-assisted synthesis of 5-mono and 1,5-disubstituted tetrazoles. Mendeleev Comm. 2011, 21, 334–336. [Google Scholar] [CrossRef]
- Epishina, M.A.; Kulikov, A.S.; Ignat´ev, N.V.; Schulte, M.; Makhova, N.N. Synthesis of 5-alkyl-2-amino-1,3,4-thiadiazoles and α,ω-bis(2-amino-1,3,4-thiadiazol-5-yl)alkanes in ionic liquids. Mendeleev Comm. 2011, 21, 331–333. [Google Scholar] [CrossRef]
- Simirskaya, N.I.; Ignat’ev, N.V.; Schulte, M.; Zlotin, S.G. Mannich synthesis of acetylenic amino alcohols in aqueous ionic liquids. Mendeleev Comm. 2012, 22, 317–319. [Google Scholar] [CrossRef]
- Kryshtal, G.V.; Zhdankina, G.M.; Ignat’ev, N.V.; Schulte, M.; Zlotin, S.G. The orthoester Johnson-Claisen rearrangement of allylic terpenols in the presence of acidic ionic liquid. J. Fluorine Chem. 2016, 183, 23–29. [Google Scholar] [CrossRef]
- Epishina, M.A.; Kulikov, A.S.; Ignat´ev, N.V.; Schulte, M.; Makhova, N.N. Nucleophilic aromatic cine-substitution of hydrogen: The ionic liquid-promoted von Richter reaction. Mendeleev Comm. 2015, 25, 41–43. [Google Scholar] [CrossRef]
- Epishina, M.A.; Kulikov, A.S.; Ignat´ev, N.V.; Schulte, M.; Makhova, N.N. Efficient synthesis of tertiary acyclic amides by the Chapman rearrangement of aryl benzimidates in ionic liquids. Mendeleev Comm. 2015, 25, 126–128. [Google Scholar] [CrossRef]
- Simirskaya, N.I.; Ignat’ev, N.V.; Schulte, M.; Zlotin, S.G. Unprecedented acceleration of the domino reaction between methyl 4-hydroxyalk-3-ynoates and amines in ionic liquids. Mendeleev Comm. 2011, 21, 94–96. [Google Scholar] [CrossRef]
- Serguchev, Y.A.; Lourie, L.F.; Ponomarenko, M.V.; Rusanov, E.B.; Ignat’ev, N.V. Fluorolactonization of unsaturated carboxylic acids with F-TEDA-BF4 in ionic liquids. Tetrahedron Lett. 2011, 52, 5166–5169. [Google Scholar] [CrossRef]
- Epishina, M.A.; Kulikov, A.S.; Struchkova, M.I.; Ignat´ev, N.V.; Schulte, M.; Makhova, N.N. Ionic liquids-assisted synthesis of 3,4-dihydroisoquinolines by the Bishler-Napieralski reaction. Mendeleev Comm. 2012, 22, 267–269. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Aleksandrova, N.S.; Ignat´ev, N.V.; Schulte, M. Straightforward one-pot synthesis of benzofuroxans from o-halonitrobenzenes in ionic liquids. Mendeleev Comm. 2012, 22, 95–97. [Google Scholar] [CrossRef]
- Horváth, A.; Dávid Frigyes, D.; Mahó, S.; Berente, Z.; Kollár, L.; Skoda-Földes, R. Facile synthesis of steroidal vicinal hydroxysulfides via the reaction of steroidal epoxides with thiols in the presence of an ionic liquid. Synthesis 2009, 2009, 4037–4041. [Google Scholar]
- Yang, M.-H.; Yan, G.-B.; Zheng, Y.-F. Regioselective ring-opening reactions of 1,2-epoxides with thiols and arylselenols directly promoted by [Bmim]BF4. Tetrahedron Lett. 2008, 49, 6471–6474. [Google Scholar] [CrossRef]
- Ranu, B.C.; Mandal, T.; Banerjee, S.; Dey, S.S. Ionic liquid promoted regio- and stereo-selective thiolysis of epoxides—A simple and green approach to β-hydroxy- and β-keto sulfides. Aust. J. Chem. 2007, 60, 278–283. [Google Scholar] [CrossRef]
- Chen, J.; Wu, H.; Jin, C.; Zhang, X.; Xie, Y.; Su, W. Highly regioselective ring-opening of epoxides with thiophenols in ionic liquids without the use of any catalyst. Green Chem. 2006, 8, 330–332. [Google Scholar] [CrossRef]
- Tanini, D.; Angeli, A.; Capperucci, A. Ionic liquids as an alternative reaction medium for HMDST based synthesis of thioaldehydes. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 156–158. [Google Scholar] [CrossRef]
- Yu, H.; Dong, D.; Ouyang, Y.; Wang, Y.; Liu, Q. NaOH-promoted thiolysis of oxiranes using 2-[bis(alkylthio)methylene]-3-oxo-N-o-tolylbutanamides as odorless thiol equivalents. Synlett 2007, 2007, 151–155. [Google Scholar] [CrossRef]
- Lenardão, E.J.; Santi, C.; Sancineto, L. New Frontiers in Organoselenium Compounds; Springer: New York, NY, USA, 2018. [Google Scholar]
- Tanini, D.; Capperucci, A. Synthesis and applications of organic selenols. Adv. Synth. Catal. 2021, 363, 5360–5385. [Google Scholar] [CrossRef]
- Capperucci, A.; Petrucci, A.; Faggi, C.; Tanini, D. Click reaction of selenols with isocyanates: Rapid access to selenocarbamates as peroxide-switchable reservoir of thiolperoxidase-like catalysts. Adv. Synth. Catal. 2021, 363, 4256–4263. [Google Scholar] [CrossRef]
- Xu, H.; Cao, W.; Zhang, X. Selenium-containing polymers: Promising biomaterials for controlled release and enzyme mimics. Acc. Chem. Res. 2013, 46, 1647–1658. [Google Scholar] [CrossRef]
- Domazetovic, V.; Fontani, F.; Tanini, D.; D’Esopo, V.; Viglianisi, C.; Marcucci, G.; Panzella, L.; Napolitano, A.; Brandi, M.L.; Capperucci, A.; et al. Protective role of benzoselenophene derivatives of resveratrol on the induced oxidative stress in intestinal myofibroblasts and osteocytes. Chem. Biol. Interact. 2017, 275, 13–21. [Google Scholar] [CrossRef]
- Tanini, D.; Capperucci, A.; Ferraroni, M.; Carta, F.; Angeli, A.; Supuran, C.T. Direct and straightforward access to substituted alkyl selenols as novel carbonic anhydrase inhibitors. Eur. J. Med. Chem. 2020, 185, 1118112. [Google Scholar] [CrossRef]
- Angeli, A.; Carta, F.; Donnini, S.; Capperucci, A.; Ferraroni, M.; Tanini, D.; Supuran, C.T. Selenolesterase enzyme activity of carbonic anhydrases. Chem. Commun. 2020, 56, 4444–4447. [Google Scholar] [CrossRef]
- Barchielli, G.; Capperucci, A.; Tanini, D. The role of selenium in pathologies: An updated review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Tanini, D.; Lupori, B.; Malevolti, G.; Ambrosi, M.; Lo Nostro, P.; Capperucci, A. Direct biocatalysed synthesis of first sulfur-, selenium- and tellurium-containing L-ascorbyl hybrid derivatives with radical trapping and GPx-like properties. Chem. Commun. 2019, 55, 5705–5708. [Google Scholar] [CrossRef] [Green Version]
- Sabir, S.; Kumar, G.; Prakash Verma, V.; Jat, J.L. Aziridine ring opening: An overview of sustainable methods. ChemistrySelect 2018, 3, 3702–3711. [Google Scholar] [CrossRef]
- Namutebi, M.; McGarrigle, E.M.; Aggarwal, V.K. Ring-opening of NH-aziridines with thiols in ionic liquids: Application to the synthesis of aminosulfide catalysts for asymmetric epoxidation of aldehydes. Phosphorus Sulfur Silicon Relat. Elemen. 2010, 185, 1250–1272. [Google Scholar] [CrossRef]
- Salman, S.M.; Narayanaperumal, S.; Schwab, R.S.; Bender, C.R.; Rodrigues, O.E.D.; Dornelles, L. CuO nano particles and [bmim]BF4: An application towards the synthesis of chiral β-seleno amino derivatives via ring opening reaction of aziridines with diorganyl diselenides. RSC Adv. 2012, 2, 8478–8482. [Google Scholar] [CrossRef]
- Salman, S.M.; Schwab, R.S.; Alberto, E.E.; Vargas, J.; Dornelles, L.; Rodrigues, O.E.D.; Braga, A.L. Efficient ring opening of protected and unprotected aziridines promoted bystable zinc selenolate in ionic liquid ring opening of protected and unprodtected aziridines. Synlett 2011, 2011, 69–72. [Google Scholar]
- Tanini, D.; Borgogni, C.; Capperucci, A. Mild and selective silicon-mediated access toenantioenriched 1,2-mercaptoamines and β-amino arylchalcogenides. New J. Chem. 2019, 43, 6388–6393. [Google Scholar] [CrossRef]
- Tanini, D.; Barchielli, G.; Benelli, F.; Degl’Innocenti, A.; Capperucci, A. Aziridines ring opening by silyl chalcogenides: A stereoselective access to polyfunctionalized molecules as precursor of sulfurated and selenated heterocycles. Phosphorus Sulfur Silicon Relat. Elemen. 2015, 190, 1265–1270. [Google Scholar] [CrossRef]
- Xu, J. Synthesis of four- to seven-membered heterocycles by ring expansion: Ring expansions of thiiranes and thietanes. In Topics in Heterocyclic Chemistry; D’hooghe, M., Ha, H.J., Eds.; Springer: Cham, Switzerland, 2015; Volume 41, pp. 311–361. [Google Scholar] [CrossRef]
- Kudo, H.; Sato, K.; Nishikubo, T. Controlled insertion reaction of thiirane into carbamothioate: Novel synthesis of well-defined polysulfide. Macromolecules 2010, 43, 9655–9659. [Google Scholar] [CrossRef]
- Repetto, E.; Manzano, V.E.; Uhrig, M.L.; Varela, O. Synthesis of branched dithiotrisaccharides via ring-opening reaction of sugar thiiranes. J. Org. Chem. 2012, 77, 253–265. [Google Scholar] [CrossRef]
- Chen, L.; Capone, D.L.; Jeffery, D.W. Analysis of potent odour-active volatile thiols in foods and beverages with a focus on wine. Molecules 2019, 24, 2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, H.; Nakajima, Y. Novel SN2 ring-opening reactions of 2- and 2,2-substituted thiiranes with thiols using Na1-exchanged X-type zeolite or triethylamine in methanol. J. Chem. Soc. Perkin Trans. 2 1998, 2441–2446. [Google Scholar] [CrossRef]
- Fokin, A.V.; Kolomiets, A.F.; Rudnitskaya, L.S.; Shevehenko, V.I. Reaction for nucleophilic opening of thiirane ring by thiols. Russ. Chem. Bull. 1975, 24, 582–584. [Google Scholar] [CrossRef]
- Vargas, J.; Narayanaperumal, S.; Gul, K.; Ravanello, B.B.; Dornelles, L.; Soares, L.C.; Alves, C.F.S.; Schneider, T.; Vaucher, R.D.A.; Santos, R.C.V.; et al. Synthesis of chiral β-chalcogen amine derivatives and Gram-positive bacteria activity. Tetrahedron 2012, 68, 10444–10448. [Google Scholar] [CrossRef]


 | |||||
| Entry | Ionic Liquid | Catalyst | Time | Yield (%) a | |
| 3a | 4a | ||||
| 1 | [bmim][BF4] | TBAF·xH2O (20%) | 2 h | 58 b | - |
| 2 | [bmim][BF4] | PhONnBu4 (40%) | 4 h | 51 b | - |
| 3 | [bmim][BF4] | - | 12 h | 10 c | 13 |
| 4 | [bmim][BF4] | - | 48 h | 24 d,e,f | 27 |
| 5 | [bmim][PF6] | TBAF·xH2O (20%) | 3 h | 47 b,e | - |
| 6 | [bmim][PF6] | - | 3 h | 28 c,e,f | 22 |
 | |||||
| Entry | R | Catalyst | Time | Product | Yield (%) a,b |
| 1 | CH2OiPr (±)-1a | TBAF·xH2O (20%) | 3 h |  | 58 |
| 2 | CH2OBn (S)-(+)-1b | TBAF·xH2O (20%) | 3 h |  | 63 |
| 3 | CH2OBn (S)-(+)-1b | - | 26 h |  | 44 |
| 4 | CH3 (±)-1c | TBAF·xH2O (20%) | 2 h |  | 39 |
| 5 | CH3 (±)-1c | - | 26 h |  | 14 |
| 6 | C6H5 (±)-1d | TBAF·xH2O (20%) | 3.5 h |   | 61 (3d) 11 (5) |
 | |||||
| Entry | Ionic Liquid | Catalyst/T(°C) | Time | 3a:4a | Yield (%) a,b |
| 1 | [emim][msu] | -/rt | 2 h | 1:1.2 c | 73 |
| 2 | [emim]atf] | -/rt | 2 h | 1:1.6 c | 78 |
| 3 | [bmpl][dca] | -/rt | 2 h | 1:1.6 c | 78 |
| 4 | [emim][otf] | -/rt | 4 h | > 1:9 c | 16 |
| 5 | [emim][otf] | TBAF·xH2O d/rt | 2 h | > 9:1 | 28 |
| 6 | [hmim][ntf] | -/rt | 3 h | > 1:9 c | 27 |
| 7 | [hmim][ntf] | TBAF·xH2O d/rt | 2 h | > 9:1 | 66 |
| 8 | [hmim][ntf] | -/70 °C | 6 h | 1:1.4 c | 65 |
| 9 | [hmim][fap] | TBAF·xH2O d/rt | 2 h | > 9:1 | 52 |
| 10 | [bmpl][ntf] | TBAF·xH2O d/rt | 1.5 h | > 9:1 | 58 |
| 11 | [bmpl][ntf] | -/70 °C | 3 h | 1:1.7 c | 63 |
| 12 | [bmpl][fap] | TBAF·xH2O d/rt | 2 h | > 9:1 | 26 |
| 13 | [bmpl][fap] | -/70 °C | 6 h | 1:1.1 | 37 |
 | ||||||
| Entry | R | R1 | Ionic Liquid | Conditions | Products | Yield (%) a,b |
| 1 | CH2OBn (R)-(-)-1b | H | [emim][msu] | r.t./2 h | 3b:4b > 10:90 | 65 (59) c,d |
| 2 | C6H5 (±)-1d | H | [emim][atf] | r.t./3.5 h | (3d,4d):(5,6) > 30:70 e | 59 (58) f |
| 3 | C6H5 (±)-1d | H | [bmpl][dca] | r.t./3.5 h | (3d,4d):(5,6) > 20:80 e | 67 (65) f |
| 4 | CH2OH (S)-(-)-1e | H | [emim][atf] | r.t./2.5 h | 3e:4e > 10:90 | 60 (57) c |
| 5 |  1f |  | [emim][msu] | TBAF·xH2O 70 °C/18 h | 3f | 38 c,g |
| 6 |  1f |  | [emim][atf] | TBAF·xH2O 18 h | 3f | 27 c,h |
| 7 |  1f |  | [bmpl][dca] | TBAF·xH2O 70 °C/18 h | 3f | <10 c,i |
 | |||||
| Entry | Ionic Liquid | Catalyst | Time | Products | Yield (%) a |
| 1 | [bmim][BF4] | - | 24 h | 12 | 8 b |
| 2 | [bmim][BF4] | TBAF·xH2O (20%) | 2 h | 7:9:11 = 1:1:1 c | 40 c |
| 3 | [emim]msu] | - | 5 h | 12:11 > 95:5 | 28 |
| 4 | [emim]msu] | TBAF·xH2O (20%) | 2 h | 9:11 = 1:3 c | 36 c |
| 5 | [emim][atf] | - | 4 h | 12:9 > 95:5 | 35 |
| 6 | [emim][atf] | TBAF·xH2O (20%) | 90 min | 9:11 > 95:5 | 33 d |
| 7 | [emim][atf]] | TBAF·xH2O (20%) | 30 min | 7:(9 + 11) > 95:5 | 56 |
| 8 | [bmpl][dca] | - | 5 h | 12:11 > 95:5 | 27 |
| 9 | [bmpl][dca] | TBAF·xH2O (20%) | 3 h | 9:11 = 1:2 c | 35 c |
 | ||||||
| Entry | R | R1 | Ionic Liquid | Conditions | 14:15 | Yield (%) a |
| 1 | CH2OiPr 1a | H | [emim][msu] | r.t./90 min | 1:1 | 72 b,c |
| 2 | CH2OiPr 1a | H | [hmim][ntf] | r.t./90 min | 2:1 | 70 b,c |
| 3 | CH2OiPr 1a | H | [hmim][fap] | r.t./90 min | 2:1 | 58 b,c |
| 4 | CH2OiPr 1a | H | [bmpl][ NTf2] | r.t./90 min | 2:1 | 64 b,c |
| 5 | CH2OiPr 1a | H | [bmim][PF6] | r.t./90 min | 1.5:1 | 72 b,c |
| 6 |  1f |  | [emim][msu] | TBAF·xH2O r.t./18 h | >99:1 | 16 d,e |
| 7 |  1f |  | [emim][atf] | TBAF·xH2O r.t./18 h | >99:1 | 12 d,e |
| 8 |  1f |  | [bmpl][dca] | 70 °C/18 h | >99:1 | <10 e |
 | ||||||
| Entry | R | Ch | Ionic Liquid | Conditions | Product | Yield (%) a |
| 1 | (CH2)2SMe | S | [bmim][PF6] | TBAF·xH2O/r.t./6 h | 20a | 48 b |
| 2 | (CH2)2SMe | S | [emim][atf] | r.t./3 h | 20ac | 57 |
| 3 | (CH2)2SMe | S | [bmpl][dca] | r.t./3 h | 20a | 54 |
| 4 | (CH2)2SMe | S | [emim][otf] | TBAF·xH2O/r.t./2 h | 20a | 63 |
| 5 | (CH2)2SMe | S | [hmim][fap] | TBAF·xH2O/r.t./2 h | 20a | 56 |
| 6 | (CH2)2SMe | S | [emim][msu] | TBAF·xH2O/r.t./3 h | 20a | 61 |
| 7 | (CH2)2SMe | S | [hmim][ntf] | TBAF·xH2O/r.t./3 h | 20a | 49 |
| 8 | (CH2)2SMe | S | [bmpl][ntf] | TBAF·xH2O/r.t./3 h | 20a | 65 |
| 9 | (CH2)2SMe | S | [bmpl][fap] | TBAF·xH2O/r.t./3 h | 20a | 43 |
| 10 | (CH2)2SMe | Se | [bmim][PF6] | TBAF·xH2O/r.t./4 h | 21ac,d | 45 |
| 11 | (CH2)2SMe | Se | [emim][msu] | r.t./3 h | 21a | 54 |
| 12 | (CH2)2SMe | Se | [hmim][fap] | r.t./3 h | 21a | 42 |
| 13 | (CH2)2SMe | Se | [bmpl][ntf] | r.t./3 h | 21a | 68 |
| 14 | i-Pr | S | [bmim][PF6] | TBAF·xH2O/r.t./4 h | 20b | 48 |
| 15 | i-Pr | S | [emim][atf] | r.t./3 h | 20bc,d | 55 |
| 16 | i-Pr | S | [bmpl][dca] | r.t./6 h | 20b | 59 e |
| 17 | i-Pr | S | [hmim][ntf] | 70°C/10 h | 20b | 47 |
| 18 | i-Pr | S | [bmpl][ntf] | TBAF·xH2O/r.t./3 h | 20b | 38 f |
| 19 | i-Pr | S | [hmim][fap] | TBAF·xH2O/r.t./2 h | 20b | 46 |
| 20 | i-Pr | Se | [bmim][PF6] | TBAF·xH2O/r.t./3 h | 21b | 47 |
 | |||
| Ionic Liquid | Time | Products | Yield (%) a |
| [bmim][PF6] | 5 h | 22 | 28 |
| [emim][atf] | 3 h | 22:23 = 4:1 | 48 b,c |
| [bmpl][dca] | 1.5 h | 22:23 = 4:1 | 52 b,c |
| [emim][msu] | 3 h | 22:23 = 3:1 | 61 b,c |
 | ||||
| Entry | Ionic Liquid | Conditions | Products | Yield (%) a,b |
| 1 | [bmim][PF6] | TBAF·xH2O/r.t./6 h | 26 | 52 |
| 2 | [emim][msu] | r.t./2 h 30 min | 25, 26 | 56 c,d |
| 3 | [emim][atf] | TBAF·xH2O/r.t./4 h | 25, 26 | 54 c,d |
| 4 | [hmim][ntf] | r.t./3 h | 25, 26 | 59 c,d |
| 5 | [bmpl][ntf] | r.t./2 h 30 min | 25, 26 | 48 c,d |
| 6 | [hmim][fap] | r.t./2 h 30 min | 25, 26 | 45 d,e |
| 7 | [bmpl][fap] | TBAF·xH2O/r.t./4 h | 25, 26 | 49 d,f,g |
| 8 | [bmpl][dcn] | r.t./2 h | 27 | 30 f |
| 9 | [emim][otf] | TBAF·xH2O/r.t./2 h | 25, 26, 27 | 57 f,h,i |
| 10 | [hmim][fap] | TBAF·xH2O/r.t./3 h | 28, 29 | 42 l.m |
| Abbreviations of Ionic Liquids | Full Name | Anions | Cations |
|---|---|---|---|
| [bmim][BF4] | 1-Butyl-3-methylimidazolium tetrafluoroborate | [BF4−] | |
| [bmim][PF6] | 1-Butyl-3-methylimidazolium hexafluorophosphate | [PF6−] | |
| [emim][otf] | 1-Ethyl-3-methylimidazolium trifluoromethanesulfonate | [CF3SO3−] | |
| [emim][msu] | 1-Ethyl-3-methylimidazolium methylsulfate | [CH3OSO3−] |  |
| [emim][atf] | 1-Ethyl-3-methylimidazolium trifluoroacetate | [CF3COO−] | (Alk = n-Butyl, Ethyl, n-Hexyl) |
| [hmim][fap] | 1-Hexyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate | [(C2F5)3PF3−] | |
| [hmim][ntf] | 1-Hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide | [N(CF3SO2)2−] | |
| [bmpl][ntf] | 1-Butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide | [N(CF3SO2)2−] | |
| [bmpl][dcn] | 1-Butyl-1-methylpyrrolidinium dicyanamide | [N(CN)2−] |  |
| [bmpl][fap] | 1-Butyl-1-methylpyrrolidinium tris(pentafluoroethyl)trifluorophosphate | [(C2F5)3PF3−] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanini, D.; Pecchi, T.; Ignat’ev, N.V.; Capperucci, A. Ionic Liquids-Assisted Ring Opening of Three-Membered Heterocycles with Thio- and Seleno-Silanes. Catalysts 2022, 12, 1259. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12101259
Tanini D, Pecchi T, Ignat’ev NV, Capperucci A. Ionic Liquids-Assisted Ring Opening of Three-Membered Heterocycles with Thio- and Seleno-Silanes. Catalysts. 2022; 12(10):1259. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12101259
Chicago/Turabian StyleTanini, Damiano, Tommaso Pecchi, Nikolai V. Ignat’ev, and Antonella Capperucci. 2022. "Ionic Liquids-Assisted Ring Opening of Three-Membered Heterocycles with Thio- and Seleno-Silanes" Catalysts 12, no. 10: 1259. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12101259