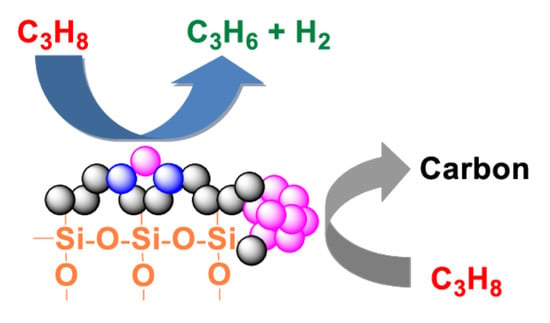
Propane Dehydrogenation on Co-N-C/SiO2 Catalyst: The Role of Single-Atom Active Sites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts Characterization
2.2. Gas-Phase Catalytic Reactions of Propylene in a Flow Reactor
3. Materials and Methods
3.1. Materials
3.2. Catalysts Preparation
3.3. Catalysts Characterization Techniques
3.4. Catalytic Performance Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmermann, H.; Walzl, R. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009. [Google Scholar]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-P.; Yang, D.; Wang, Z.; Yuan, Z.-Y. State-of-the-art catalysts for direct dehydrogenation of propane to propylene. Chin. J. Catal. 2019, 40, 1233–1254. [Google Scholar] [CrossRef]
- Martino, M.; Meloni, E.; Festa, G.; Palma, V. Propylene Synthesis: Recent Advances in the Use of Pt-Based Catalysts for Propane Dehydrogenation Reaction. Catalysts 2021, 11, 1070. [Google Scholar] [CrossRef]
- Dong, S.; Altvater, N.R.; Mark, L.O.; Hermans, I. Assessment and comparison of ordered & non-ordered supported metal oxide catalysts for upgrading propane to propylene. Appl. Catal. A-Gen. 2021, 617, 118121. [Google Scholar] [CrossRef]
- Chen, S.; Chang, X.; Sun, G.; Zhang, T.; Xu, Y.; Wang, Y.; Pei, C.; Gong, J. Propane dehydrogenation: Catalyst development, new chemistry, and emerging technologies. Chem. Soc. Rev. 2021, 50, 3315–3354. [Google Scholar] [CrossRef]
- Otroshchenko, T.; Jiang, G.; Kondratenko, V.A.; Rodemerck, U.; Kondratenko, E.V. Current status and perspectives in oxidative, non-oxidative and CO2-mediated dehydrogenation of propane and isobutane over metal oxide catalysts. Chem. Soc. Rev. 2021, 50, 473–527. [Google Scholar] [CrossRef]
- Dai, Y.; Gao, X.; Wang, Q.; Wan, X.; Zhou, C.; Yang, Y. Recent progress in heterogeneous metal and metal oxide catalysts for direct dehydrogenation of ethane and propane. Chem. Soc. Rev. 2021, 50, 5590–5630. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, P.; Yang, J.; Zhu, Y.-A.; Chen, D. C–H bond activation in light alkanes: A theoretical perspective. Chem. Soc. Rev. 2021, 50, 4299–4358. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, B.; Liu, G. Metal-based catalysts for the non-oxidative dehydrogenation of light alkanes to light olefins. React. Chem. Eng. 2021, 6, 9–26. [Google Scholar] [CrossRef]
- Liu, L.; Deng, Q.F.; Agula, B.; Zhao, X.; Ren, T.Z.; Yuan, Z.Y. Ordered mesoporous carbon catalyst for dehydrogenation of propane to propylene. Chem. Commun. 2011, 47, 8334–8336. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Sun, X.; Zhang, B.; Sun, X.; Su, D. Hybrid Nanocarbon as a Catalyst for Direct Dehydrogenation of Propane: Formation of an Active and Selective Core–Shell sp2/sp3 Nanocomposite Structure. Chem.-Eur. J. 2014, 20, 6324–6331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ge, G.; Li, W.; Guo, X.; Wang, G. Modulating the microstructure and surface chemistry of carbocatalysts for oxidative and direct dehydrogenation: A review. Chin. J. Catal. 2016, 37, 644–670. [Google Scholar] [CrossRef]
- Hu, Z.-P.; Chen, C.; Ren, J.-T.; Yuan, Z.-Y. Direct dehydrogenation of propane to propylene on surface-oxidized multiwall carbon nanotubes. Appl. Catal. A-Gen. 2018, 559, 85–93. [Google Scholar] [CrossRef]
- Hu, Z.P.; Ren, J.T.; Yang, D.; Wang, Z.; Yuan, Z.Y. Mesoporous carbons as metal-free catalysts for propane dehydrogenation: Effect of the pore structure and surface property. Chin. J. Catal. 2019, 40, 1385–1394. [Google Scholar] [CrossRef]
- Zhao, Z.; Dai, Y.; Ge, G.; Wang, G. Explosive Decomposition of a Melamine–Cyanuric Acid Supramolecular Assembly for Fabricating Defect-Rich Nitrogen-Doped Carbon Nanotubes with Significantly Promoted Catalysis. Chem. Eur. J. 2015, 21, 8004–8009. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Dai, Y.; Ge, G. Nitrogen-doped nanotubes-decorated activated carbon-based hybrid nanoarchitecture as a superior catalyst for direct dehydrogenation. Catal. Sci. Technol. 2015, 5, 1548–1557. [Google Scholar] [CrossRef]
- Song, Y.; Liu, G.; Yuan, Z.Y. N-, P- and B-doped mesoporous carbons for direct dehydrogenation of propane. RSC Adv. 2016, 6, 94636–94642. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, H.; Yang, J.; Zhao, J.; Yan, L.; Song, H.; Chou, L. Fe-containing N-doped porous carbon for isobutane dehydrogenation. Microp. Mesop. Mater. 2020, 293, 109820. [Google Scholar] [CrossRef]
- Xie, J.; Kammert, J.D.; Kaylor, N.; Zheng, J.W.; Choi, E.; Pham, H.N.; Sang, X.; Stavitski, E.; Attenkofer, K.; Unocic, R.R.; et al. Atomically Dispersed Co and Cu on N-Doped Carbon for Reactions Involving C–H Activation. ACS Catal. 2018, 8, 3875–3884. [Google Scholar] [CrossRef]
- Cao, T.; Dai, X.; Li, F.; Liu, W.; Bai, Y.; Fu, Y.; Qi, W. Efficient Non-Precious Metal Catalyst for Propane Dehydrogenation: Atomically Dispersed Cobalt-nitrogen Compounds on Carbon Nanotubes. ChemCatChem 2021, 13, 3067–3073. [Google Scholar] [CrossRef]
- Wang, Y.; Suo, Y.; Ren, J.-T.; Wang, Z.; Yuan, Z.-Y. Spatially isolated cobalt oxide sites derived from MOFs for direct propane dehydrogenation. J. Colloid Interface Sci. 2021, 594, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, W.; Ma, Z.; Yu, F.; Chen, Y.; Liao, H.; Wang, X.; Zhou, J. Highly Effective Direct Dehydrogenation of Propane to Propylene by Microwave Catalysis at Low Temperature over Co−Sn/NC Microwave Catalyst. ChemCatChem 2021, 13, 1009–1022. [Google Scholar] [CrossRef]
- Li, Y.-M.; Liu, Z.-Y.; Zhang, Q.-Y.; Wang, Y.-J.; Cui, G.-Q.; Zhao, Z.; Xu, C.-M.; Jiang, G.-Y. Influence of carbonization temperature on cobalt-based nitrogen-doped carbon nanopolyhedra derived from ZIF-67 for nonoxidative propane dehydrogenation. Pet. Sci. 2022. [Google Scholar] [CrossRef]
- Chernov, A.N.; Sobolev, V.I.; Koltunov, K.Y. Propane dehydrogenation to propylene over Co@N-doped carbon: Structure-activity-selectivity relationships. Catal. Commun. 2022, 170, 106495. [Google Scholar] [CrossRef]
- Wang, X.; Fu, H.; Li, W.; Zheng, J.; Li, X. Metal (metal = Fe, Co), N codoped nanoporous carbon for efficient electrochemical oxygen reduction. RSC Adv. 2014, 4, 37779–37785. [Google Scholar] [CrossRef]
- Morozan, A.; Goellner, V.; Nedellec, Y.; Hannauer, J.; Jaouen, F. Effect of the Transition Metal on Metal-Nitrogen-Carbon Catalysts for the Hydrogen Evolution Reaction. J. Electrochem. Soc. 2015, 162, H719–H726. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Yan, W.; Liu, X.; Yang, X.; Miao, S.; Wang, W.; Wang, A.; Zhang, T. Single-atom dispersed Co-N-C catalyst: Structure identification and performance for hydrogenative coupling of nitroarenes. Chem. Sci. 2016, 7, 5758–5764. [Google Scholar] [CrossRef] [Green Version]
- Eisenberg, D.; Slot, T.K.; Rothenberg, G. Understanding oxygen activation on metal- and nitrogen-codoped carbon catalysts. ACS Catal. 2018, 8, 8618–8629. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, E.; Hu, C.; Zhao, Y.; Zhang, H.; Zhang, Y.; Ji, M.; Yu, J.; Cong, G.; Liu, H.; et al. Controlled Synthesis of Co@N-Doped Carbon by Pyrolysis of ZIF with 2-Aminobenzimidazole Ligand for Enhancing Oxygen Reduction Reaction and the Application in Zn−Air Battery. ACS Appl. Mater. Interfaces 2020, 12, 11693–11701. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-X.; Chen, Y.-Z.; Cao, L.; Lu, J.; Giang, H.-L. Conversion of a metal–organic framework to N-doped porous carbon incorporating Co and CoO nanoparticles: Direct oxidation of alcohols to esters. Chem. Commun. 2015, 51, 8292–8295. [Google Scholar] [CrossRef]
- Gao, Y.; Han, Z.; Hong, S.; Wu, T.; Li, X.; Qiu, J.; Sun, Z. ZIF-67-Derived Cobalt/Nitrogen-Doped Carbon Composites for Efficient Electrocatalytic N2 Reduction. ACS Appl. Energy Mater. 2019, 2, 6071–6077. [Google Scholar] [CrossRef]
- Li, X.; Surkus, A.-E.; Rabeah, J.; Anwar, M.; Dastigir, S.; Junge, H.; Bruckner, A.; Beller, M. Cobalt Single-Atom Catalysts with High Stability for Selective Dehydrogenation of Formic Acid. Angew. Chem. Int. Ed. 2020, 59, 15849–15854. [Google Scholar] [CrossRef] [PubMed]
- Astrakova, T.V.; Sobolev, V.I.; Koltunov, K.Y. Facile mechanochemical synthesis of Co@NC catalysts for oxidative esterification of benzyl alcohol with methanol. Catal. Commun. 2020, 137, 105952. [Google Scholar] [CrossRef]
- Chernov, A.N.; Astrakova, T.V.; Sobolev, V.I.; Koltunov, K.Y. Liquid versus gas phase dehydrogenation of formic acid over Co@N-doped carbon materials. The role of single atomic sites. Mol. Catal. 2021, 504, 111457. [Google Scholar] [CrossRef]
- Chernov, A.N.; Astrakova, T.V.; Koltunov, K.Y.; Sobolev, V.I. Ethanol dehydrogenation to acetaldehyde over Co@N-doped carbon. Catalysts 2021, 11, 1411. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, P.; Zhang, Z.; Zhang, J.; Yang, P.; Gao, P.; Gao, J.; Liu, D. Co-N-C supported on SiO2: A facile, efficient catalyst for aerobic oxidation of amines to imines. RSC Adv. 2017, 7, 47366–47372. [Google Scholar] [CrossRef] [Green Version]
- Dewangan, N.; Ashok, J.; Sethia, M.; Das, S.; Pati, S.; Kus, H.; Kawi, S. Cobalt-Based Catalyst Supported on Different Morphologies of Alumina for Non-oxidative Propane Dehydrogenation: Effect of Metal Support Interaction and Lewis Acidic Sites. ChemCatChem 2019, 11, 4923–4934. [Google Scholar] [CrossRef]
- Dai, Y.; Gu, J.; Tian, S.; Wu, Y.; Chen, J.; Li, F.; Du, Y.; Peng, L.; Ding, W.; Yang, Y. γ-Al2O3 sheet-stabilized isolate Co2+ for catalytic propane dehydrogenation. J. Catal. 2020, 381, 482–492. [Google Scholar] [CrossRef]
- Kung, H.H. Reduction of oxides. Stud. Surf. Sci. Catal. 1989, 45, 91–109. [Google Scholar] [CrossRef]
- Golovnya, R.V.; Samusenko, A.L.; Mistryukov, E.A. Analysis of polar compounds on PEG-40M/KF glass capillary columns. J. High Resolut. Chromatogr. 1979, 2, 609–612. [Google Scholar] [CrossRef]












| Entry | Catalyst | Co (wt%) | SBET (m2 g−1) |
|---|---|---|---|
| 1 | Co-N-C/SiO2 | 9.9 | 509 |
| 2 | Co-N-C/SiO2-L | 0.8 | 424 |
| 3 | Co/SiO2 | 10.9 | 335 |
| 4 | N-C/SiO2 | - | 412 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernov, A.N.; Sobolev, V.I.; Gerasimov, E.Y.; Koltunov, K.Y. Propane Dehydrogenation on Co-N-C/SiO2 Catalyst: The Role of Single-Atom Active Sites. Catalysts 2022, 12, 1262. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12101262
Chernov AN, Sobolev VI, Gerasimov EY, Koltunov KY. Propane Dehydrogenation on Co-N-C/SiO2 Catalyst: The Role of Single-Atom Active Sites. Catalysts. 2022; 12(10):1262. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12101262
Chicago/Turabian StyleChernov, Aleksey N., Vladimir I. Sobolev, Evgeny Yu. Gerasimov, and Konstantin Yu. Koltunov. 2022. "Propane Dehydrogenation on Co-N-C/SiO2 Catalyst: The Role of Single-Atom Active Sites" Catalysts 12, no. 10: 1262. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12101262