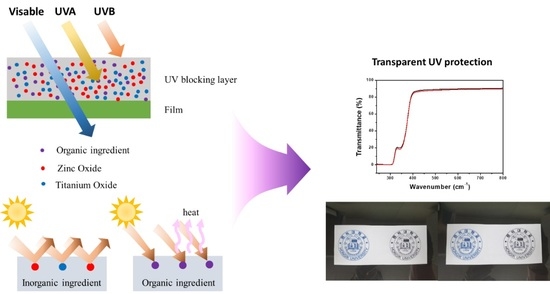
1. Introduction
Because of its great transparency, strength in mechanical use, and outstanding barrier qualities, polyethylene terephthalate (PET) is commonly used for packing liquids and freshly cut fruit. Because PET is highly transparent to both visible and ultraviolet (UV) light, it can be mixed with low molecular weight, UV-absorbing compounds to lessen the nutrient deterioration that occurs when food is exposed to UV light. UV rays break the chemical bonds of synthetic resins via photolysis, leaving them with cracks or discoloration resulting in a decline in their physical properties, such as impact and tensile strengths [
1,
2]. The properties of synthetic resins, such as polyethylene and polypropylene, can be degraded by their exposure to light, so UV protection (organic sunscreen and inorganic sunblock) is used to prevent their deterioration (decomposition and discoloration). The primary cause for the aging of synthetic resins is the UV rays of sunlight; thus, their weather and light resistances can be improved by the addition of sunscreen or sunblock. Organic sunscreen absorbs UV energy that has reached the surface of a material and often blocks UV-B (280–320 nm) radiation, whereas inorganic sunblock physically scatters UV rays and mainly employs ZnO and TiO
2 for this purpose, making it effective in blocking UV-A (320–400 nm) radiation, but it is not easy to ensure transparency, as shown in
Figure 1A [
3,
4]. Among the organic ultraviolet absorbers, benzotriazole, which has a good absorption effect of near-UV, light stabilizer, and synergistic effect, is the most widely used, and benzophenone, cyanoacrylate, and triazine are used according to characteristics (
Figure 1B); therefore, both the blocking agents are used together to effectively defend against UV rays.
Plants create their own sunscreen, such as sinapoyl malate, to prevent the UV rays of the sun from damaging their DNA and hindering their growth. Sinapoyl malate has a ring structure similar to those of common sunscreen components. Stavros et al. found that when oxybenzone molecules—an organic sunscreen—absorb light and become excited, 90% of them decompose and emit heat, but 10% remain excited without breaking; this is similar to a phosphorescence phenomenon, in which light is applied to an object, and is then removed for a long time. By manipulating the rotation area or vibrating the material to convert the excess energy into heat, the material can be returned to a stable ground state [
5].
A UV absorber is a synthetic resin additive that selectively absorbs UV energy and converts it into thermal energy; benzotriazole is mainly used for this because it has the best UV absorption ability and does not stain synthetic resins, thereby giving rise to color stability over a long period of time. A representative product for this purpose is Tinuvin. It has already been reported that standard Tinuvin
® UV absorbers are used in food and packaging containers available in the market, with no known health issues associated with their use [
6]. The requirements of a UV absorber are good light absorption in the UV absorption wavelength range (280–400 nm), thermal and chemical stability, low volatility, excellent compatibility with the target substance, light stability of the absorbent itself, transparency, odorlessness, nontoxicity, and a small amount for effective use. A UV absorber absorbs light in the 250–400 nm wavelength range and converts it into thermal energy.
ZnO, which has excellent UV-A and UV-B properties, and TiO
2, which is more specialized in blocking UV-B, are white powders used in medicines, pigments, and cosmetic raw materials. ZnO is a UV protection material recognized by the U.S. Food and Drug Administration for its stability and effectiveness and is widely used in antibacterial products due to its ecofriendliness, excellent thermal stability, low toxicity to the human body, and strong resistance to bacteria and fungi [
7]. In addition, TiO
2 has the ability to absorb light to induce photo-oxidation–reduction reactions via an electron transition and photocatalytically degrade bacteria and organic matter; therefore, it is used to develop antibacterial functional packaging materials to maintain food quality and prevent its decay [
8]. Studies have reported that fine nanosized TiO
2 particles show high scattering of UV rays, making TiO
2 an excellent material for use in UV protection applications [
9]. Diaz–Visurraga et al. found that chitosan films with 17–170 nm-sized TiO
2 exhibit increased light absorption toward both UV-A and UV-B, thereby showing superior UV protection compared to nondoped films [
10]. To ensure transparency, as well as UV protection for maintaining the quality of products, nanoparticles with size <100 nm must be uniformly dispersed [
11]. The factor that determines visible light transmittance and UV protection properties is the size of the dispersed particles; nanoparticle materials have very good reactivity properties because of their high specific surface area [
12]. In addition, Sabzi et al. demonstrated that TiO
2, which was surface-treated with the coupling agent amino propyl dimethoxy silane to efficiently disperse nanosized TiO
2, has an improved UV protection effect compared with its pretreatment state [
13]. In this study, we developed a coating material that is transparent and exhibits guaranteed UV protection via two synthesis methods by using ZnO, TiO
2, and Tinuvin; it was confirmed that the silane-based coupling coating suppresses the photocatalytic activity of ZnO and TiO
2, thereby improving their dispersibility and safety.
2. Results and Discussion
As the size of ZnO and TiO
2 particles decreases, the transparency of the visible light region increases; hence, these materials were synthesized under two sets of synthesis conditions, and their particle shapes were observed by using SEM analysis, with the results shown in
Figure 2. As can be observed in
Figure 2A, the ZnO–TiO
2–TEA sample exhibits a wirelike structure with a size of several nanometers (195–252 nm). To widen the surface area of ZnO, the nanotetrapod approach can be considered [
14,
15]. In comparison, the ZnO–TiO
2 nanocomposite exhibits the particles like structures with a size of several hundred nanometers (61–124 nm), as shown in
Figure 2B.
Figure 2C shows the morphology of a ZnO–TiO
2–silane composite, which confirms that the ZnO–TiO
2 surface was completely coated with silane; moreover, EDX spectroscopy and elemental mapping results confirmed the presence of Si in the composites.
As shown in
Figure 3A, all of the samples were synthesized ZnO particles with a hexagonal wurtzite crystal structure with XRD peaks of (2θ, reflection plane) = (31.8°, (100)), (34.5°, (002)), (36.3°, (101)), (47.5°, (102)) and (56.6°, (110)) and were uniformly distributed with TiO
2 in three different phases. The prominent peak of TiO
2 was consistent with the anatase TiO
2 phase (2θ = 25°, (101)), and crystals of the rutile (2θ = 27.5°, (100)) and brookite (2θ = 31°, (120)) TiO
2 phases were also observed. In the case of TiO
2, the average particle size of the samples was estimated by applying the value of the main XRD peak centered at approximately 25° to the Debye–Scherrer equation (D = 0.9λ/(βcosθ)) [
16]. In this equation, D is the average crystallite size, the constant with a value of 0.9 is the shape factor, λ is the wavelength of the incident X-rays (1.5406 Å), β is the full width at half maximum, and θ is the diffraction angle of the maximum peak. The average crystallite sizes of ZnO and TiO
2 are 75.45 and 68.24 nm, respectively.
Figure 3B shows the FTIR spectra in the wavenumber range of 500–4000 cm
−1 of the samples for (a) ZnO–TiO
2–TEA, (b) ZnO–TiO
2 and (c) ZnO–TiO
2–silane. Intense and weak bands appearing below 1000 cm
−1 are due to O–Ti–O bonding [
17]. The sample of
Figure 3B(b,c) shows stronger O-Ti-O bonding at a wavenumber of 563 cm
−1 than
Figure 3B(a). The peaks for C–H at 1000–1100 and 2898 cm
−1 are due to the absorption of alkane functional groups [
18]. The function of C=C and C=C–C vibrations can be observed at 1635 and 1447 cm
−1, respectively [
19]. The peaks at 1627 and 2370 cm
−1 correspond to the H–O–H vibrations in absorbed water molecules [
20]. The sample of ZnO–TiO
2–TEA exhibits a more intense absorption hydroxyl peak (–OH) than that of ZnO–TiO
2.
Figure 4 shows the dependence of the spectroscopic characteristics of ZnO–TiO
2–TEA and ZnO–TiO
2 on the silane coupling agent and Tinuvin. The silane coupling agent blocks UV-A transmittance by increasing the absorption in the UV-A region, whereas, Tinuvin increases the absorption in both the UV-A and UV-B regions, thereby significantly lowering the transmittance for all UV regions, e.g., lowering it to 20% for UV-A. Samples obtained via the synthesis of ZnO–TiO
2–TEA exhibit a transmittance of ≤2% in both the UV-A and UV-B regions, but it is difficult to ensure transparency as the transmittance is limited to 25% for visible light. Samples using Tinuvin, ZnO, and TiO
2 entirely block UV-B, 80% UV-A, and 80% visible light, thereby ensuring their transparency. In addition, it can be seen that the sample treated with a silane coupling exhibits excellent UV-blocking of the ZnO and TiO
2 photocatalysts when exposed to UV rays, as shown in the spectra in
Figure 4C. The easiest way to protect against UV rays is to increase the concentration of UV blockers. This is because according to the Beer–Lambert law
(
A: absorbance,
T: transmittance,
P0: incident radiant power,
P: transmitted radiant powder,
ε: molar absorptivity,
b: path length of sample,
c: concentration of absorber) [
21], the greater the thickness and concentration of UV blockers is, the higher is the absorption rate of light; however, if the concentration increases, it is difficult to ensure transmittance to visible light, and the material becomes harmful to skin. Therefore, the use of small amounts of ZnO, TiO
2, and Tinuvin serves as an environmentally friendly and transparent sunscreen.
2.1. Mechanism for Silane Coupling ZnO–TiO2 UV Blocker
Surface modification allows changes to be made to a material and control of its surface properties to improve its limitations.
Figure 5 shows the contact angles before and after the silane coupling of the ZnO and TiO
2 powders, as well as the optical microscope images at 40× magnification. Moreover, the samples were coated with PET film, and optical microscopy images at 10 and 40× magnifications and contact angle measurements of the films were obtained. The contact angle of the powder changed from superhydrophilic at <2° to hydrophobic at 134° ± 2.1° by surface treatment via silane coupling. The effect of the silane coating on the powder is noticeable, but when a film is incorporated, the effect of the film on the contact angle is unclear. Optical microscopy was used to observe the powder distribution on a film to observe the silane coating effect. The film using the silane-coated sample was very evenly dispersed, whereas, the film using the non-silane-coated sample featured lumps of powder.
Electrons (e
−) and holes (h
+) generate on the surface of ZnO and TiO
2 under UV radiation when used as photocatalysts; the e
− reacts with oxygen on the photocatalyst surface to form
. The h
+ reacts with moisture in the air to form hydroxyl radicals (–OH) which can oxidize and decompose powerful organic substances, such as odorous compounds, viruses, and bacteria, by converting them into H
2O and CO
2 [
22]. However, due to their superhydrophilicity, the hydroxyl groups aggregate and clump together; therefore, to improve the dispersibility of the pigment, it is surface-treated with a coupling agent, which improves the wettability of the organic solvent by removing hydroxyl groups, as shown in
Figure 6.
2.2. Mechanism of Tinuvin UV Absorber
Polymer degradation is inhibited in the field of polymer science by screening out incident UV radiation with UV absorbers [
23]. Tinuvin is one of many benzotriazoles, which also include UV absorption properties and exceptional photostability, that are used as UV absorbers. By being exposed to UV radiation, the derivatives induce excited-state intramolecular proton transfer (ESIPT) and produce zwitterionic structures that aid in nonradiative deactivation as shown in
Figure 7 [
24]. Because there is a deactivation pathway that passes through the conical junction, the internal conversion following ESIPT occurs very quickly, making it challenging to deconstruct these derivatives during the limited lifetimes of the excited states [
25]. Damage-causing UV light is preferentially absorbed by UV absorbers made of benzotriazoles, and through ESIPT-based nonradiative deactivation, it is then released as thermal energy. Metal-free organic dyes can benefit from the idea of UV absorbers stabilizing and protecting macromolecules.
3. Materials and Methods
3.1. Materials
ZnAc (zinc acetate dihydrate, Zn[C2H3O2]2·2H2O, 99.5%), ZnO (zinc oxide, 99.5%), and TiO2 (titanium oxide, 99.5%) powders were purchased from Daejung Chemical & Material Co. Ltd., Republic of Korea and used to synthesize UV-blocking agents. Pure ethanol and TEA (triethanolamine, C6H15NO3) were used as a solvent and stabilizer, respectively. For the surface modification of ZnO and TiO2 powders, 3Cl-TMS (3-chloropropyl trimethoxy silane, Cl(CH2)3Si(OCH3)3, 97%, Sigma-Aldrich, Merck Korea, Seoul, Republic of Korea) was used as a silane coupling agent and MEK (methyl ethyl ketone, C4H8O, 99%, Daejung, Republic of Korea) was used as a solvent. Propylene glycol monomethyl ether acetate (PGMEA, C6H12O3, Sigma-Aldrich) was used without purification as a dispersion solvent. The polyethylene terephthalate (PET) film is a thermoplastic polymer that can be amorphous, crystalline, or a mixture of both depending on how it is processed. The PET film used as a substrate was purchased as a commercial A4-sized film with the dimensions 210 × 297 mm and a thickness of 100 μm from the LamiAce company, Seoul, Republic of Korea.
3.2. Synthesis and Surface Treatment Method
Synthesis of ZnO–TiO
2–TEA: A mixture of ZnAc and TEA was prepared in a ZnAc:TEA molar ratio 5:2 [
26]. TEA was mixed in a reaction vessel with 40 mL of ethanol and stirred by using a magnetic stirrer before the addition of ZnAc and further stirring of the resulting mixture at 50–60 °C for 30 min. TiO
2 equal to Zn molar ratio of ZnAc was mixed and stirred for 15 min, to which 10 mL of deionized water was added and stirred for 30 min. PET film was washed in a sonicator for 10 min in a mixture of ethanol and acetone; this film was then coated via dipping method, and dried in an oven at 60 °C for 6 h.
Synthesis of ZnO–TiO2: ZnO and TiO2 powders were mixed in a mass ratio of 1:1 and ground by a ball milling, which was performed at 175 rpm for 4 h, where, in addition to the powders, the ball mill was filled with an appropriate amount of alumina balls and ethanol. After the completion of the milling and removal of the alumina balls, the sample was washed with ethanol and covered with aluminum foil to prevent it being contaminated with impurities, before being left to air dry for a day to allow evaporation of the ethanol. The resulting powder was then dried in the oven at 80 °C for 3 h.
Silane coating: MEK (300 mL) and 3Cl-TMS (9 g) were introduced into a 500 mL reaction vessel, which was well sealed to prevent solvent evaporation and ingress of impurities before being sonicated for 30 min. ZnO and TiO2 samples (60 g) obtained via the ZnO-TiO2 synthesis were added to the above solution, and the mixture was stirred uniformly and slowly at room temperature (20–25 °C) for 6 h. When the stirring was completed, the remaining impurities and unreacted substances were removed by washing with ethanol, and then the product was dried in the oven at 70 °C for 24 h to obtain ZnO–TiO2–silane.
Tinuvin mixing: The ZnO–TiO2 composite was mixed in 9:1 ratio with PGMEA, to which 5% wt thermosetting coating solution based on the composite weight was added and coated on the PET film. Similarly, the ZnO–TiO2–silane–Tinuvin composite was synthesized via the addition of ZnO–TiO2–silane.
3.3. Measurement and Characteristic Analysis
The crystal phase and morphology of the samples were compared and analyzed by using an X-ray diffraction (XRD; D8 ADVANCE, Bruker, Billerica, MA, USA) and field emission scanning electron microscopy (FE-SEM; Merlin Compact, Carl Zeiss, Jena, Thuringia, Germany), respectively. The structure and surface characteristics of the surface-modified samples were analyzed by using Fourier-transform infrared (FTIR; Spectrum X, Perkin Elmer, Waltham, MA, USA) and energy-dispersive X-ray (EDX; Aztec Energy X-MaxN, Oxford instruments, Abingdon, Oxfordshire, England) spectrometers. In addition, the spectroscopic characteristics of the dispersed film coatings were obtained by using a diffusion reflectance ultraviolet-visible-near-infrared (UV–vis –NIR) spectrometer (SolidSpec-3700, Shimadzu, Kyoto, Kansai, Japan). The contact angles of the film and powder were measured by using a contact angle goniometer (L2004A1, Ossila, Sheffield, South Yorkshire, UK), and the powder dispersibility of the film was measured by using a microscope (LCD MICRO 5MP, Bresser, Rhede, North Rhine-Westphalia, Germany).