Direct Z-Scheme g-C3N5/Cu3TiO4 Heterojunction Enhanced Photocatalytic Performance of Chromene-3-Carbonitriles Synthesis under Visible Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Prepared Nanomaterials
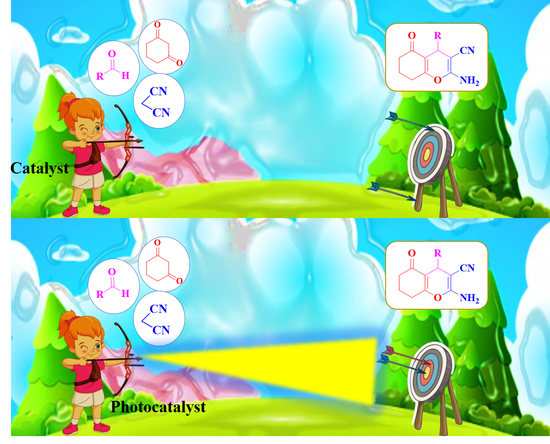
2.2. Photocatalytic Activity of g-C3N5/Cu3TiO4 Nanocomposite
2.3. Proposed Mechanism of 1.4a Synthesis
2.4. Transport Process of Photo-Excited Charge Carriers
3. Materials and Methods
3.1. Materials Information
3.2. Preparation of Citrus Limon Extract
3.3. Preparation of Cu3TiO4 NPs
3.4. Preparation of g-C3N5 Nanosheets and Composite
3.5. General Synthesis of Carbonitriles (1.4a-g) under Visible Light
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nazeri, M.T.; Shaabani, A. Synthesis of Polysubstituted Pyrroles via Isocyanide-Based Multicomponent Reactions as an Efficient Synthesis Tool. New J. Chem. 2021, 45, 21967–22011. [Google Scholar] [CrossRef]
- Chopra, P.K.P.G.; Lambat, T.L.; Mahmood, S.H.; Chaudhary, R.G.; Banerjee, S. Sulfamic Acid as Versatile Green Catalyst Used for Synthetic Organic Chemistry: A Comprehensive Update. Chem. Sel. 2021, 6, 6867–6889. [Google Scholar] [CrossRef]
- Dekamin, M.G.; Eslami, M. Highly Efficient Organocatalytic Synthesis of Diverse and Densely Functionalized 2-Amino-3-Cyano-4H-Pyrans under Mechanochemical Ball Milling. Green Chem. 2014, 16, 4914–4921. [Google Scholar] [CrossRef]
- Wang, X.; Yin, F.; Bi, Y.; Cheng, G.; Li, J.; Hou, L.; Li, Y.; Yang, B.; Liu, W.; Yang, L. Rapid and Sensitive Detection of Zika Virus by Reverse Transcription Loop-Mediated Isothermal Amplification. J. Virol. Methods 2016, 238, 86–93. [Google Scholar] [CrossRef]
- Singh, S.B. Copper Nanocatalysis in Multi-Component Reactions: A Green to Greener Approach. Curr. Catal. 2018, 7, 80–88. [Google Scholar] [CrossRef]
- Ghamari Kargar, P.; Bagherzade, G. Robust, Highly Active, and Stable Supported Co(ii) Nanoparticles on Magnetic Cellulose Nanofiber-Functionalized for the Multi-Component Reactions of Piperidines and Alcohol Oxidation. RSC Adv. 2021, 11, 23192–23206. [Google Scholar] [CrossRef]
- Kamanna, K.; Khatavi, S.Y. Microwave-Accelerated Carbon-Carbon and Carbon-Heteroatom Bond Formation via Multi-Component Reactions: A Brief Overview. Curr. Microw. Chem. 2020, 7, 23–39. [Google Scholar] [CrossRef]
- Ramesh, R.; Maheswari, S.; Malecki, J.G.; Lalitha, A. NaN3 Catalyzed Highly Convenient Access to Functionalized 4 H -Chromenes: A Green One-Pot Approach for Diversity Amplification. Polycycl. Aromat. Compd. 2020, 40, 1581–1594. [Google Scholar] [CrossRef]
- Majumdar, N.; Paul, N.D.; Mandal, S.; de Bruin, B.; Wulff, W.D. Catalytic Synthesis of 2 H -Chromenes. ACS Catal. 2015, 5, 2329–2366. [Google Scholar] [CrossRef]
- Hauguel, C.; Ducellier, S.; Provot, O.; Ibrahim, N.; Lamaa, D.; Balcerowiak, C.; Letribot, B.; Nascimento, M.; Blanchard, V.; Askenatzis, L.; et al. Design, Synthesis and Biological Evaluation of Quinoline-2-Carbonitrile-Based Hydroxamic Acids as Dual Tubulin Polymerization and Histone Deacetylases Inhibitors. Eur. J. Med. Chem. 2022, 240, 114573. [Google Scholar] [CrossRef]
- Khan, S.A.; Asiri, A.M.; Basisi, H.M.; Asad, M.; Zayed, M.E.M.; Sharma, K.; Wani, M.Y. Synthesis and Evaluation of Quinoline-3-Carbonitrile Derivatives as Potential Antibacterial Agents. Bioorg. Chem. 2019, 88, 102968. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.B.; Kumari, P. A Review: Structure-Activity Relationship and Antibacterial Activities of Quinoline Based Hybrids. J. Mol. Struct. 2022, 1268, 133634. [Google Scholar] [CrossRef]
- Liu, T.; Wu, Z.; He, Y.; Xiao, Y.; Xia, C. Single and Dual Target Inhibitors Based on Bcl-2: Promising Anti-Tumor Agents for Cancer Therapy. Eur. J. Med. Chem. 2020, 201, 112446. [Google Scholar] [CrossRef]
- Sharma, D.; Kumar, M.; Das, P. Application of Cyclohexane-1,3-Diones for Six-Membered Oxygen-Containing Heterocycles Synthesis. Bioorg. Chem. 2021, 107, 104559. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Pinto, S.; Pontes, O.; Baltazar, F.; Costa, M. In Vivo Efficacy Studies of Chromene-Based Compounds in Triple-Negative Breast Cancer—A Systematic Review. Eur. J. Pharmacol. 2020, 887, 173452. [Google Scholar] [CrossRef]
- Costa, M.; Dias, T.A.; Brito, A.; Proença, F. Biological Importance of Structurally Diversified Chromenes. Eur. J. Med. Chem. 2016, 123, 487–507. [Google Scholar] [CrossRef]
- Sadek, K.U.; Mekheimer, R.A.H.; Abd-Elmonem, M.; Abdel-Hameed, A.; Elnagdi, M.H. Recent Developments in the Enantioselective Synthesis of Polyfunctionalized Pyran and Chromene Derivatives. Tetrahedron Asymmetry 2017, 28, 1462–1485. [Google Scholar] [CrossRef]
- Chaudhary, A.; Singh, K.; Verma, N.; Kumar, S.; Kumar, D.; Sharma, P.P. Chromenes—A Novel Class of Heterocyclic Compounds: Recent Advancements and Future Directions. Mini.-Rev. Med. Chem. 2022, 22, 2736–2751. [Google Scholar] [CrossRef]
- Son, Y.-A.; Gwon, S.-Y.; Kim, S.-H. Chromene and Imidazole Based D-π-A Chemosensor Preparation and Its Anion Responsive Effects. Mol. Cryst. Liq. Cryst. 2014, 599, 16–22. [Google Scholar] [CrossRef]
- Dagilienė, M.; Markuckaitė, G.; Krikštolaitytė, S.; Šačkus, A.; Martynaitis, V. Cyanide Anion Determination Based on Nucleophilic Addition to 6-[(E)-(4-Nitrophenyl)Diazenyl]-1′,3,3′,4-Tetrahydrospiro[Chromene-2,2′-Indole] Derivatives. Chem. Sens. 2022, 10, 185. [Google Scholar] [CrossRef]
- Kar, S.; Sanderson, H.; Roy, K.; Benfenati, E.; Leszczynski, J. Green Chemistry in the Synthesis of Pharmaceuticals. Chem. Rev. 2022, 122, 3637–3710. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Kamanna, K.; Amaregouda, Y. Synthesis of Bioactive Scaffolds Catalyzed by Agro-Waste-Based Solvent Medium. Phys. Sci. Rev. 2022. [Google Scholar] [CrossRef]
- Védrine, J. Heterogeneous Catalysis on Metal Oxides. Catalysts 2017, 7, 341. [Google Scholar] [CrossRef] [Green Version]
- Chellapandi, T.; Madhumitha, G. Montmorillonite Clay-Based Heterogenous Catalyst for the Synthesis of Nitrogen Heterocycle Organic Moieties: A Review. Mol. Divers. 2021. [CrossRef] [PubMed]
- Singh, B.K.; Lee, S.; Na, K. An Overview on Metal-Related Catalysts: Metal Oxides, Nanoporous Metals and Supported Metal Nanoparticles on Metal Organic Frameworks and Zeolites. Rare Met. 2020, 39, 751–766. [Google Scholar] [CrossRef]
- Shifrina, Z.B.; Matveeva, V.G.; Bronstein, L.M. Role of Polymer Structures in Catalysis by Transition Metal and Metal Oxide Nanoparticle Composites. Chem. Rev. 2020, 120, 1350–1396. [Google Scholar] [CrossRef]
- Terna, A.D.; Elemike, E.E.; Mbonu, J.I.; Osafile, O.E.; Ezeani, R.O. The Future of Semiconductors Nanoparticles: Synthesis, Properties and Applications. Mater. Sci. Eng. B 2021, 272, 115363. [Google Scholar] [CrossRef]
- Ge, M.Z.; Cao, C.Y.; Li, S.H.; Tang, Y.X.; Wang, L.N.; Qi, N.; Lai, Y.K. In situ plasmonic Ag nanoparticle anchored TiO2 nanotube arrays as visible-light-driven photocatalysts for enhanced water splitting. Nanoscale 2016, 8, 5226–5234. [Google Scholar] [CrossRef]
- Ge, M.; Cai, J.; Iocozzia, J.; Cao, C.; Huang, J.; Zhang, X.; Lin, Z. A review of TiO2 nanostructured catalysts for sustainable H2 generation. Int. J. Hydrogren Energy 2017, 42, 8418–8449. [Google Scholar] [CrossRef]
- Ge, M.; Li, Q.; Cao, C.; Huang, J.; Li, S.; Zhang, S.; Lai, Y. One-dimensional TiO2 nanotube photocatalysts for solar water splitting. Adv. Sci. 2017, 4, 1600152. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Luo, T.; Lin, Y.; Yang, M. Construction of Novel Z-Scheme g-C3N4/AgBr-Ag Composite for Efficient Photocatalytic Degradation of Organic Pollutants under Visible Light. Catalysts 2022, 12, 1309. [Google Scholar] [CrossRef]
- Cuong, H.N.; Pansambal, S.; Ghotekar, S.; Oza, R.; Thanh Hai, N.T.; Viet, N.M.; Nguyen, V.-H. New Frontiers in the Plant Extract Mediated Biosynthesis of Copper Oxide (CuO) Nanoparticles and Their Potential Applications: A Review. Environ. Res. 2022, 203, 111858. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Choudhary, P.; Kumar, A.; Camargo, P.H.C.; Krishnan, V. Recent Advances in Plasmonic Photocatalysis Based on TiO2 and Noble Metal Nanoparticles for Energy Conversion, Environmental Remediation, and Organic Synthesis. Small 2022, 18, 2101638. [Google Scholar] [CrossRef]
- Gnanasekaran, L.; Pachaiappan, R.; Kumar, P.S.; Hoang, T.K.A.; Rajendran, S.; Durgalakshmi, D.; Soto-Moscoso, M.; Cornejo-Ponce, L.; Gracia, F. Visible Light Driven Exotic p (CuO)–n (TiO2) Heterojunction for the Photodegradation of 4-Chlorophenol and Antibacterial Activity. Environ. Pollut. 2021, 287, 117304. [Google Scholar] [CrossRef]
- Marzo, L.; Pagire, S.K.; Reiser, O.; König, B. Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew. Chem. Int. Ed. 2018, 57, 10034–10072. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef]
- Arunachalapandi, M.; Roopan, S.M. Environment Friendly G-C3N4-Based Catalysts and Their Recent Strategy in Organic Transformations. High Energy Chem. 2022, 56, 73–90. [Google Scholar] [CrossRef]
- Mun, S.J.; Park, S.-J. Graphitic Carbon Nitride Materials for Photocatalytic Hydrogen Production via Water Splitting: A Short Review. Catalysts 2019, 9, 805. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Fang, S.; Shen, Q.; Fan, J.; Li, Q.; Lv, K. Recent Advances of Doping and Surface Modifying Carbon Nitride with Characterization Techniques. Catalysts 2022, 12, 962. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. A Critical Review on Graphitic Carbon Nitride (g-C3N4)-Based Materials: Preparation, Modification and Environmental Application. Coord. Chem. Rev. 2022, 453, 214338. [Google Scholar] [CrossRef]
- Mane, G.P.; Talapaneni, S.N.; Lakhi, K.S.; Ilbeygi, H.; Ravon, U.; Al-Bahily, K.; Mori, T.; Park, D.H.; Vinu, A. Highly ordered nitrogen-rich mesoporous carbon nitrides and their superior performance for sensing and photocatalytic hydrogen generation. Angew. Chem. Int. Ed. 2017, 56, 8481–8485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Yao, J.; Chen, S.; Zhang, J.; Li, R.; Peng, T. Construction of rGO-coupled C3N4/C3N5 2D/2D Z-scheme heterojunction to accelerate charge separation for efficient visible light H2 evolution. Appl. Catal. B. 2022, 318, 121822. [Google Scholar] [CrossRef]
- Luo, T.; Hu, X.; She, Z.; Wei, J.; Feng, X.; Chang, F. Synergistic effects of Ag-doped and morphology regulation of graphitic carbon nitride nanosheets for enhanced photocatalytic performance. J. Mol. Liq. 2021, 324, 114772. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Liu, Y.; Wang, C.; Yan, R.; Chen, X. Constructing Cd0.5Zn0.5S/Bi2WO6 S-scheme heterojunction for boosted photocatalytic antibiotic oxidation and Cr (VI) reduction. Adv. Powder Technol. 2023, 2, 100073. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wang, S.; Yang, F.; Zhou, W. Mesoporous black TiO2/MoS2/Cu2S hierarchical tandem heterojunctions toward optimized photothermal-photocatalytic fuel production. J. Chem. Eng. 2022, 427, 131830. [Google Scholar] [CrossRef]
- Wang, S.; Guan, B.Y.; Lou, X.W.D. Construction of ZnIn2S4–In2O3 hierarchical tubular heterostructures for efficient CO2 photoreduction. J. Am. Chem. Soc. 2018, 140, 5037–5040. [Google Scholar] [CrossRef]
- Xiao, Y.; Guo, S.; Tian, G.; Jiang, B.; Ren, Z.; Tian, C.; Li, W.; Fu, H. Synergetic enhancement of surface reactions and charge separation over holey C3N4/TiO2 2D heterojunctions. Sci. Bull. 2021, 66, 275–283. [Google Scholar] [CrossRef]
- Humayun, M.; Ullah, H.; Tahir, A.A.; bin Mohd Yusoff, A.R.; Mat Teridi, M.A.; Nazeeruddin, M.K.; Luo, W. An Overview of the Recent Progress in Polymeric Carbon Nitride Based Photocatalysis. Chem. Rec. 2021, 21, 1811–1844. [Google Scholar] [CrossRef]
- Palanivel, B.; Mani, A. Conversion of a Type-II to a Z-Scheme Heterojunction by Intercalation of a 0D Electron Mediator between the Integrative NiFe2O4/g-C3N4 Composite Nanoparticles: Boosting the Radical Production for Photo-Fenton Degradation. ACS Omega 2020, 5, 19747–19759. [Google Scholar] [CrossRef]
- Lu, L.; Wang, G.; Zou, M.; Wang, J.; Li, J. Effects of Calcining Temperature on Formation of Hierarchical TiO2/g-C3N4 Hybrids as an Effective Z-Scheme Heterojunction Photocatalyst. Appl. Surf. Sci. 2018, 441, 1012–1023. [Google Scholar] [CrossRef]
- Wang, M.; Jin, C.; Kang, J.; Liu, J.; Tang, Y.; Li, Z.; Li, S. CuO/g-C3N4 2D/2D Heterojunction Photocatalysts as Efficient Peroxymonosulfate Activators under Visible Light for Oxytetracycline Degradation: Characterization, Efficiency and Mechanism. Chem. Eng. J. 2021, 416, 128118. [Google Scholar] [CrossRef]
- Shen, D.; Li, X.; Ma, C.; Zhou, Y.; Sun, L.; Yin, S.; Huo, P.; Wang, H. Synthesized Z-Scheme Photocatalyst ZnO/g-C3N4 for Enhanced Photocatalytic Reduction of CO2. New J. Chem. 2020, 44, 16390–16399. [Google Scholar] [CrossRef]
- Meng, J.; Wang, X.; Liu, Y.; Ren, M.; Zhang, X.; Ding, X.; Guo, Y.; Yang, Y. Acid-Induced Molecule Self-Assembly Synthesis of Z-Scheme WO3/g-C3N4 Heterojunctions for Robust Photocatalysis against Phenolic Pollutants. Chem. Eng. J. 2021, 403, 126354. [Google Scholar] [CrossRef]
- Dadigala, R.; Bandi, R.; Gangapuram, B.R.; Dasari, A.; Belay, H.H.; Guttena, V. Fabrication of Novel 1D/2D V2O5/g-C3N4 Composites as Z-Scheme Photocatalysts for CR Degradation and Cr (VI) Reduction under Sunlight Irradiation. J. Environ. Chem. Eng. 2019, 7, 102822. [Google Scholar] [CrossRef]
- Wu, B.; Li, Y.; Su, K.; Tan, L.; Liu, X.; Cui, Z.; Yang, X.; Liang, Y.; Li, Z.; Zhu, S.; et al. The Enhanced Photocatalytic Properties of MnO2/g-C3N4 Heterostructure for Rapid Sterilization under Visible Light. J. Hazard. Mater. 2019, 377, 227–236. [Google Scholar] [CrossRef]
- He, R.; Zhou, J.; Fu, H.; Zhang, S.; Jiang, C. Room-Temperature in Situ Fabrication of Bi2O3/g-C3N4 Direct Z-Scheme Photocatalyst with Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2018, 430, 273–282. [Google Scholar] [CrossRef]
- Yin, H.; Cao, Y.; Fan, T.; Zhang, M.; Yao, J.; Li, P.; Chen, S.; Liu, X. In Situ Synthesis of Ag3PO4/C3N5 Z-Scheme Heterojunctions with Enhanced Visible-Light-Responsive Photocatalytic Performance for Antibiotics Removal. Sci. Total Environ. 2021, 754, 141926. [Google Scholar] [CrossRef]
- Vadivel, S.; Fujii, M.; Rajendran, S. Facile Synthesis of Broom Stick like FeOCl/g-C3N5 Nanocomposite as Novel Z-Scheme Photocatalysts for Rapid Degradation of Pollutants. Chemosphere 2022, 307, 135716. [Google Scholar] [CrossRef]
- Chu, W.; Li, S.; Zhou, H.; Shi, M.; Zhu, J.; He, P.; Liu, W.; Wu, M.; Wu, J.; Yan, X. A Novel Bionic Flower-like Z-Scheme Bi4O5I2/g-C3N5 Heterojunction with I−/I3− active sites and π-conjugated structure for increasing photocatalytic oxidation of elemental mercury. J. Environ. Chem. Eng. 2022, 10, 108623. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, K.; Zhong, X.; Jiang, F. Z-Scheme LaCoO3/C3N5 for Efficient Full-Spectrum Light-Simulated Solar Photocatalytic Hydrogen Generation. RSC Adv. 2022, 12, 24026–24036. [Google Scholar] [CrossRef]
- Rajendran, S.; Chellapandi, T.; UshaVipinachandran, V.; Venkata Ramanaiah, D.; Dalal, C.; Sonkar, S.K.; Madhumitha, G.; Bhunia, S.K. Sustainable 2D Bi2WO6/g-C3N5 Heterostructure as Visible Light-Triggered Abatement of Colorless Endocrine Disruptors in Wastewater. Appl. Surf. Sci. 2022, 577, 151809. [Google Scholar] [CrossRef]
- Deng, S.; Li, Z.; Zhao, T.; Huang, G.; Wang, J.; Bi, J. Direct Z-Scheme Covalent Triazine-Based Framework/Bi2WO6 Heterostructure for Efficient Photocatalytic Degradation of Tetracycline: Kinetics, Mechanism and Toxicity. J. Water Process Eng. 2022, 49, 103021. [Google Scholar] [CrossRef]
- Das, A. LED Light Sources in Organic Synthesis: An Entry to a Novel Approach. Lett. Org. Chem. 2022, 19, 283–292. [Google Scholar] [CrossRef]
- Cai, Z.; Huang, Y.; Ji, H.; Liu, W.; Fu, J.; Sun, X. Type-II Surface Heterojunction of Bismuth-Rich Bi4O5Br2 on Nitrogen-Rich g-C3N5 Nanosheets for Efficient Photocatalytic Degradation of Antibiotics. Sep. Purif. Technol. 2022, 280, 119772. [Google Scholar] [CrossRef]
- Arunachalapandi, M.; Roopan, S.M. Ultrasound/Visible Light-Mediated Synthesis of N-Heterocycles Using g-C3N4/Cu3TiO4 as Sonophotocatalyst. Res. Chem. Intermed. 2021, 47, 3363–3378. [Google Scholar] [CrossRef]
- Wei, W.; Gong, H.; Sheng, L.; Wu, H.; Zhu, S.; Feng, L.; Li, X.; You, W. Highly Efficient Photocatalytic Activity and Mechanism of Novel Er3+ and Tb3+ Co-Doped BiOBr/g-C3N5 towards Sulfamethoxazole Degradation. Ceram. Int. 2021, 47, 24062–24072. [Google Scholar] [CrossRef]
- Vadivel, S.; Fujii, M.; Rajendran, S. Novel S-Scheme 2D/2D Bi4O5Br2 Nanoplatelets/g-C3N5 Heterojunctions with Enhanced Photocatalytic Activity towards Organic Pollutants Removal. Environ. Res. 2022, 213, 113736. [Google Scholar] [CrossRef]
- Ramezanalizadeh, H.; Rafiee, E. Design, Fabrication, Electro- and Photoelectrochemical Investigations of Novel CoTiO3/CuBi2O4 Heterojunction Semiconductor: An Efficient Photocatalyst for the Degradation of DR16 Dye. Mater. Sci. Semicond. Process. 2020, 113, 105055. [Google Scholar] [CrossRef]
- Li, M.; Lu, Q.; Liu, M.; Yin, P.; Wu, C.; Li, H.; Zhang, Y.; Yao, S. Photsoinduced Charge Separation via the Double-Electron Transfer Mechanism in Nitrogen Vacancies g-C3N5/BiOBr for the Photoelectrochemical Nitrogen Reduction. ACS Appl. Mater. Interfaces 2020, 12, 38266–38274. [Google Scholar] [CrossRef]
- Rajendran, R.; Vignesh, S.; Suganthi, S.; Raj, V.; Kavitha, G.; Palanivel, B.; Shkir, M.; Algarni, H. G-C3N4/TiO2/CuO S-Scheme Heterostructure Photocatalysts for Enhancing Organic Pollutant Degradation. J. Phys. Chem. Solids 2022, 161, 110391. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Maheskumar, V.; Yea, Y.; Yoon, Y.; Muthuraj, V.; Park, C.M. 2D/2D nitrogen-rich graphitic carbon nitride coupled Bi2WO6 S-scheme heterojunction for boosting photodegradation of tetracycline: Influencing factors, intermediates, and insights into the mechanism. Compos. Part B Eng. 2022, 234, 109726. [Google Scholar] [CrossRef]
- Chen, L.; Bao, S.; Yang, L.; Zhang, X.; Li, B.; Li, Y. Cheap Thiamine Hydrochloride as Efficient Catalyst for Synthesis of 4H-Benzo[b]Pyrans in Aqueous Ethanol. Res. Chem. Intermed. 2017, 43, 3883–3891. [Google Scholar] [CrossRef]
- Tabassum, S.; Govindaraju, S.; Kendrekar, P. (Mes-Acr-Me)+ClO4– Catalyzed Visible Light-Supported, One-Pot Green Synthesis of 1,8-Naphthyridine-3-Carbonitriles. Top. Catal. 2022, 1–9. [Google Scholar] [CrossRef]
- Aditya, M.N.; Chellapandi, T.; Prasad, G.K.; Venkatesh, M.J.P.; Khan, M.M.R.; Madhumitha, G.; Roopan, S.M. Biosynthesis of Rod Shaped Gd2O3 on G-C3N4 as Nanocomposite for Visible Light Mediated Photocatalytic Degradation of Pollutants and RSM Optimization. Diam. Relat. Mater. 2022, 121, 108790. [Google Scholar] [CrossRef]
- Mondal, M.; Banerjee, S.; Mal, S.; Das, S.; Pradhan, S.K. Nanocomposites of GaBr3 and BiBr3 Nanocrystals on BiOBr for the Photocatalytic Degradation of Dyes and Tetracycline. ACS Appl. Nano. Mater. 2022, 5, 15676–15691. [Google Scholar] [CrossRef]
- Deng, S.; Yang, Z.; Lv, G.; Zhu, Y.; Li, H.; Wang, F.; Zhang, X. WO3 Nanosheets/g-C3N4 Nanosheets’ Nanocomposite as an Effective Photocatalyst for Degradation of Rhodamine B. Appl. Phys. A 2019, 125, 44. [Google Scholar] [CrossRef]
- Miao, Z.; Zhang, Y.; Wang, N.; Xu, P.; Wang, X. BiOBr/Bi2S3 Heterojunction with S-Scheme Structureand Oxygen Defects: In-Situ Construction and Photocatalytic Behavior for Reduction of CO2 with H2O. J. Colloid Interface Sci. 2022, 620, 407–418. [Google Scholar] [CrossRef]
- Xing, P.; Zhou, F.; Li, Z. Preparation of WO3/g-C3N4 Composites with Enhanced Photocatalytic Hydrogen Production Performance. Appl. Phys. A 2019, 125, 788. [Google Scholar] [CrossRef]
- Ye, R.; Fang, H.; Zheng, Y.-Z.; Li, N.; Wang, Y.; Tao, X. Fabrication of CoTiO3/g-C3N4 Hybrid Photocatalysts with Enhanced H2 Evolution: Z-Scheme Photocatalytic Mechanism Insight. ACS Appl. Mater. Interfaces 2016, 8, 13879–13889. [Google Scholar] [CrossRef]









| S.No | Catalyst | Solvent | Conditions | Time (minutes) | Yield a (%) |
|---|---|---|---|---|---|
| 1. | CuO | EtOH | Light | 60 | 62 |
| 2. | CuOAc | EtOH | Light | 60 | 66 |
| 3. | TiO2 | EtOH | Light | 60 | 33 |
| 4. | Cu3TiO4 | EtOH | Light | 60 | 75 |
| 5. | 5% CN5CT | EtOH | Light | 3 | 67 |
| 6. | 10% CN5CT | EtOH | Light | 3 | 94 |
| 7. | 20% CN5CT | EtOH | Light | 3 | 81 |
| 8. | 30% CN5CT | EtOH | Light | 3 | 89 |
| 9. | 10% CN5CT | H2O | Light | 10 | 61 |
| 10. | 10% CN5CT | THF | RT | 60 | 23 |
| 11. | 10% CN5CT | Acetonitrile | RT | 60 | 21 |
| 12 | 10%CN5CT | EtOH | RT | 10 | 95 |
| 13. | Without Catalyst | EtOH | RT | 10 | Trace |
| 14. | 10% CN5CT | Solvent free | Heat | 10 | 78 |
 | Name | R | Time | TON a | TOF a | Yield b | Ref. c |
| 1.4a | -Ph | 5 | 865 | 3.52 | 85 | [73] | |
| 1.4b | 4-(OCH3)-Ph | 5 | 832 | 3.26 | 82 | [73] | |
| 1.4c | 2-NO2-Ph | 3 | 876 | 3.78 | 88 | [73] | |
| 1.4d | 4-OH-Ph | 5 | 831 | 3.22 | 81 | [73] | |
| 1.4e | 4-F-Ph | 5 | 878 | 3.86 | 90 | [73] | |
| 1.4f | 2-Cl-Ph | 3 | 923 | 4.63 | 94 | [73] | |
| 1.4g | 2-Nap | 3 | 902 | 4.12 | 92 | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arunachalapandi, M.; Chellapandi, T.; Madhumitha, G.; Manjupriya, R.; Aravindraj, K.; Roopan, S.M. Direct Z-Scheme g-C3N5/Cu3TiO4 Heterojunction Enhanced Photocatalytic Performance of Chromene-3-Carbonitriles Synthesis under Visible Light Irradiation. Catalysts 2022, 12, 1593. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12121593
Arunachalapandi M, Chellapandi T, Madhumitha G, Manjupriya R, Aravindraj K, Roopan SM. Direct Z-Scheme g-C3N5/Cu3TiO4 Heterojunction Enhanced Photocatalytic Performance of Chromene-3-Carbonitriles Synthesis under Visible Light Irradiation. Catalysts. 2022; 12(12):1593. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12121593
Chicago/Turabian StyleArunachalapandi, Murugan, Thangapandi Chellapandi, Gunabalan Madhumitha, Ravichandran Manjupriya, Kumar Aravindraj, and Selvaraj Mohana Roopan. 2022. "Direct Z-Scheme g-C3N5/Cu3TiO4 Heterojunction Enhanced Photocatalytic Performance of Chromene-3-Carbonitriles Synthesis under Visible Light Irradiation" Catalysts 12, no. 12: 1593. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12121593