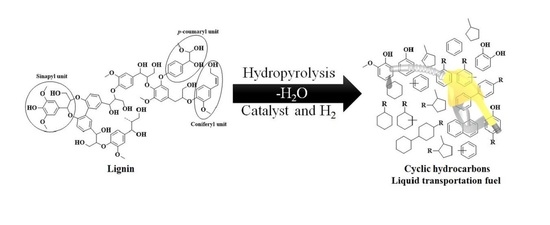
Catalytic Hydropyrolysis of Lignin for the Preparation of Cyclic Hydrocarbon-Based Biofuels
Abstract
:1. Introduction
2. Lignin Depolymerization Methods
- (i).
- Lignin depolymerization in the presence of water medium at a suitable temperature (100–350 °C) is hydrolysis. The hydrolysis of lignin may occur using acid or base catalysts. Several acidic homogenous (H2SO4, HNO3, HCl, heteropoly acids, ionic liquids, etc.) and heterogeneous (acidic metal oxide, zeolites, acidic resins/clays, sulfonated carbon materials, etc.) catalysts were extensively reported for acid-catalyzed hydrolysis/depolymerization of lignin. Biphasic medium, water, along with organic solvent or supercritical fluids were also reported for better conversion of lignin. Phenol, alkylphenols, methoxyphenols, alkenylphenols, alkyl/methoxy-phenols, and alkenyl/methoxy-phenols are primarily observed during the lignin hydrolysis [24].
- (ii).
- Lignin depolymerization in the presence of a solvent or a mixture of solvents at suitable temperatures is known as solvolysis. The process minimizes the formation of char and usually forms low-oxygen-containing aromatic hydrocarbons. Several organic solvents such as alcohols (methanol, ethanol, propanol, iso-propanol), oxanes (dioxane), ketones (acetone), alkanes (dodecane), etc., and a mixture thereof with water were frequently used for lignin solvolysis. The supported metal (Pt, Ru, Rh, Pd, Ni, Mo, Cu, and Co) catalysts displayed good yields of biooil (80–95%) [25].
- (iii).
- Thermal decomposition at high temperatures (300–1000 °C) under an inert atmosphere is called pyrolysis. Lignin pyrolysis ended up with gases (hydrogen, methane, carbon monoxide, and carbon dioxide), liquids (oxygenated cyclic hydrocarbons, especially hydroxy, methoxy, and alkyl-substituted benzenes), condensable vapors, and solid char. The thermal decomposition at high temperatures under H2 atmosphere is known as hydropyrolysis. Several supported metal catalysts were reported for the hydropyrolysis of lignin and, within this, hydrogenation and hydrodeoxygenation reaction plays an important role in obtaining high yield of non-oxygenated aromatic compounds. Syringol and guaiacol type molecules (from hardwood), guaiacol type phenols (from softwood), and a mixture of phenol, guaiacol, and syringol type compounds (from herbaceous) were predominantly observed during the pyrolysis. The non-catalytic pyrolysis-derived concoction is associated with poor stability and undergoes repolymerization to produce sticky material. Thus, the catalytic pyrolysis process improves the yield of biooil, and catalytic depolymerization produces the most stable products where there is less possibility for repolymerization [26].
- (iv).
- Oxidative cleavage of lignin in the presence of oxidants is known as oxidative lignin depolymerization. Several oxidants, such as O2, KMnO4, H2O2, and peroxy acids, etc., were reported for this type of reaction. The oxidative cleavage of lignin generally produces oxygenated-aromatic hydrocarbons (hydroxyl, carbonyl, and carboxyl-substituted benzenes). The oxygen radical species, obtained from oxidants during the reaction, perform multiple cleavages of the lignin and this leads to the formation of several oxygenated aromatic compounds [24,27].
- (v).
- Gasification is usually referred to a process of oxygen-deficient thermal decomposition of carbonaceous substances such as coal, petroleum, or lignocellulosic biomass with a major objective of producing valuable gaseous products such as hydrogen or syngas. Lignin gasification provides an array of hydrocarbons (gas, liquid, and solid) depending upon on the gasification temperature. The C/O ratio of lignin is high as compared to lignocellulose and other biomass sources, and thus formation of solid/tar, i.e., high-molecular-weight condensable organic hydrocarbons, usually take place [28].
3. Lignocellulose-Based Emerging Biofuels
4. Lignin Conversion in the Presence of H2
- Alkene chain double hydrogenation, hydrodemethoxylation (hydrogenolysis of C–OCH3) to 4-propylphenol; reaction conditions: Ni/SBA-15, 260 °C, 30 bar H2 [41].
- Alkene chain double hydrogenation, hydrodealkylation (hydrogenolysis of C–C) to guaiacol; reaction conditions: Pt/γ-Al2O3, 300 °C, H2 [44].
- Alkene chain double hydrogenation, hydrodealkylation (hydrogenolysis of C–C), hydrodemethoxylation (hydrogenolysis of C–OCH3) to phenol; reaction conditions: Pt/γ-Al2O3, 300 °C, H2 [44].
- Alkene chain double hydrogenation, hydrodealkylation (hydrogenolysis of C–C), hydrodehydroxylation (hydrogenolysis of C–OH) to anisole; reaction conditions: Pt/HY, 250 °C, 40 bar H2 [45].
- Alkene chain double hydrogenation, hydrodealkylation (hydrogenolysis of C–C), hydrodemethoxylation (hydrogenolysis of C–OCH3), hydrodehydroxylation (hydrogenolysis of C–OH) to benzene; reaction conditions: Ni/HZSM-5, 350 °C, 20 bar H2 [46].
- Alkene chain double hydrogenation, aromatic ring hydrogenation to 2-methoxy-4-propylcyclohexanol; reaction conditions: Pd/C, 250 °C, 30 bar H2 [47].
- Alkene chain double hydrogenation, aromatic ring hydrogenation, hydrodehydroxylation (hydrogenolysis of C–OH) to 1-methoxy-3-propylcyclohexane; reaction conditions: Ni/MCM-4+HZSM-5, 200 °C, 50 bar H2 [51].
- Alkene chain double hydrogenation, aromatic ring hydrogenation, hydrodemethoxylation (hydrogenolysis of C–OCH3), hydrodehydroxylation (hydrogenolysis of C–OH) to propylcyclohexane; reaction conditions: Ru/CNT, 220 °C, 50 bar H2 [52].
5. Hydropyrolysis of Lignin into Cyclic Hydrocarbons
- Chemical treatment: a. dilute acid (HCl, HNO3, and H2SO4) treatment minimizes the ash content and removes the elements (Na, K, S, P, Si, Cl, Mg, Fe, Al, etc.); b. base treatment isolates the lignin from lignocellulose biomass and improves the quality of the oil. Moreover, chemical pre-treatment weakens the linkages in the lignocellulose biomass, which easily breaks down during pyrolysis and hydrodeoxygenation processes.
- The physical treatment such as size reduction using milling or grinding of biomass usually provides a high yield of biooil. Also, heat and mass transfer are high in this case which leads to good cleavage capacity.
- The pretreatment of biomass with the hydrothermal process using a Soxhlet extractor to remove the colorants, pectin, dust particles, and elements, etc., the treated biomass provide with good-quality and low-viscosity biooil.
- Biological pretreatment is also one of the gateways to produce highly selective biooil; for example, the white-rot-fungus-treated biomass forms selectively aromatic hydrocarbons and suppresses the coke yield. In fact, the white-rot fungus has the capability to disconnect the refractory cell walls of lignocellulose.
5.1. Non-Catalytic Hydropyrolysis of Lignin
5.2. Catalytic Hydropyrolysis of Lignin
5.2.1. Acid-catalyzed Hydropyrolysis of Lignin
| S. No | Source | Catalyst | Reaction Conditions | Yield of Biooil(%) | Ref. |
|---|---|---|---|---|---|
| 1 | Poplar wood-derived lignin | HZSM-5 (Si/Al-30) | Pyrolysis at 600 °C, 20 s, He, catalyst to lignin ratio (2:1) | 35.7 mg/g of aromatic hydrocarbon | [12] |
| 2 | Kraft lignin | 1. Natural zeolite 2. HZSM-5 | Pyrolysis in two stage-reactor 1. 500 °C, N2, catalyst to lignin ratio (1:1) 2. 600 °C, N2 | 30–35% of biooil with mixture of oxygenated and non-oxygenated aromatic compounds | [68] |
| 3 | Acid-treated lignin (derived from peanut shells) | HZSM-5 | Pyrolysis at 600 °C, N2, catalyst to lignin (1:2) | 43–45% of biooil (50% sel. of aromatic hydrocarbons) | [20] |
| 4 | Kraft lignin | HZSM-5 | Pyrolysis at 600 °C, 10 min, N2, catalyst to lignin (1:1) | Yield of biooil is not reported but selectivity towards the methoxy/alkyl aromatics hydrocarbons (50% sel.) | [10] |
| 5 | Kraft lignin | HZSM-5 | Hydropyrolysis at 450 °C, 10 bar H2 for 60 min. | 47% yield of biooil (50 wt.% of aromatic hydrocarbons) | [11] |
| 6 | Kraft lignin | HY and FeReOx/ZrO2 | Hydropyrolysis (using HY) followed by hydrodeoxygenation (using FeReOx/ZrO2) | 75% selectivity towards phenolic compounds | [77] |
5.2.2. Supported Metal Catalyzed Hydropyrolysisof Lignin
- I.
- Disclosed hydropyrolysis of pyrolytic lignin using Ru/C at 400 °C, 4 h, 100 bar H2, which resulted in high liquid product yield, i.e., 75%, of which monomeric compounds comprise 50% (Table 2, entry 3). Several products, including 18–20% phenolic compounds (phenol, cresols, methoxyphenols, alkylphenols), 14–15% aromatic hydrocarbons (benzene alkylbenzenes, xylenes, naphthalene, and methylnaphthalene), and 6–7% cycloalkanes (cyclohecane, alkylcyclohexanes, alkylcyclopentanes), were identified as the major products in this work [35].
- II.
- Screened various catalysts (Ru/C, Ru/Al2O3, Ru/TiO2, Pd/C, Pd/Al2O3, Cu/ZrO2) for the hydrotreatment of Alcell lignin at 400 °C and 100 bar H2. From the studied catalysts, the Ru/TiO2 showed a high yield of biooil (78%), comprising alkylphenolics (9 wt.%), aromatic hydrocarbons (2.5 wt.%), and hydroxyphenols (3.5 wt.%) (Table 2, entry 4) [84].
- III.
- Reported hydropyrolysis of Kraft lignin with NiMo and CoMo metallic catalysts on different supports: acidic (Al2O3 and ZSM-5), neutral (activated carbon), and basic (MgO-La2O3). The hydropyrolysis was carried out at 350 °C, 100 bar H2 for 4 h. The basic sulfide NiMo/MgO-La2O3 catalyst showed high catalytic activity to obtain greater monomeric cyclic hydrocarbon yield (26 wt.%), consisting majorly of alkyl-phenolic compounds (Table 2, entry 5). The neutral activated carbon supported metal catalysts showed moderate conversion of lignin, and acidic supports (Al2O3 and ZSM-5) were found to be less active in the conversion because in the presence of acidic support re-condensation between reaction intermediates is initiated, which results in the solid residue [15].
- IV.
- Low-priced catalysts, i.e., Limonite ((FeO(OH)·nH2O)),were investigated for the conversion of Kraft lignin at 450 °C, 100 bar H2 for 4 h. The catalyst resulted in ~34% of low-molecular-weight aromatic hydrocarbons (phenol, alkylphenols and alkylbenzenes, alkylnaphthalenes, and alkyl anthracene) and a trace amount of cycloalkanes. The recycled Limonite catalyst (after the first run) was pre-treated at 450 °C and used for the second cycle, which showed a good yield of aromatic hydrocarbons with 25% yield. The authors concluded that Limonite is a cheap and potential catalyst for making bio-based phenolics and aromatics from lignin through the hydrodeoxygenation reaction [85].
- V.
- Investigated catalytic (Ru/C, CoMo/alumina, phosphided NiMO/C) hydrodeoxygenation of Kraft lignin in two-step hydropyrolysis, and as they observed a high yield of monomeric phenolic compounds (alkylphenols) as compared with single pyrolysis [86].
- VI.
- Reported several metal (Ni. Mo, W, NiMo, NiW) phosphide catalysts supported on activated carbon for the hydropyrolysis of Kraft lignin at 400–500 °C, 100 bar H2 for 4 h. The 10NiMoP/AC catalyst showed a 71% yield of biooil (with 100% mass balance) as compared with other catalysts. Moreover, the catalyst yielded (45.7 wt.%) high monomeric aromatic hydrocarbons such as alkylphenols and alkyl aromatics. The authors did not observe corresponding peaks of methoxy groups and C–O–C linkages of biooil (obtained via hydrotreating of the Kraft lignin using the NiMoP/AC catalyst), suggesting that common ether linkages were broken, leading to the formation of monomeric aromatic hydrocarbons [61].
- VII.
- Phosphided NiMo on different supports were reported for hydropyrolysis of lignin at 400 °C, 100 bar H2 for 2 h. The catalytic supports used in this work are activated carbon, SiO2-Al2O3, SiO2, MgO-Al2O3, and TiO2. Among them, the NiMoP supported on SiO2 produced a high yield (68%) of biooil, 52% monomeric compounds including 31% alkylphenols, 8% aliphatic compounds, and 5.7% aromatic compounds, etc. The SiO2 support consists of medium acidity which produced a higher yield than the basic- (MgO-Al2O3) and high-acid supports (SiO2-Al2O3). However, a reason behind the high activity of NiMoP/SiO2 is not reported [2].
| S. No | Source | Catalyst | Reaction Conditions | Yield of Biooil (%) | Ref. |
|---|---|---|---|---|---|
| 1 | Kraft and Organosolv lignin | Pd/C and Ni-Mo/aluminosilica | Hydropyrolysis at 350–450 °C, 120 bar H2 | 81% (using Pd/C) and 66% (using Ni-Mo/aluminosilica) yield of biooil | [81,82] |
| 2 | Lignin derived from hybrid poplar | Pd/HZSM-5 | Hydropyrolysis at 650 °C and 17 bar H2, catalyst-to-lignin ratio of 20:1 | 45% yield of aromatic hydrocarbons | [83] |
| 3 | Pyrolytic lignin | Ru/C | Hydropyrolysis at 400 °C, 100 bar H2 for 4 h | 75% yield of biooil (50 wt.% of monomeric compounds) | [35] |
| 4 | Alcelllignin | Ru/TiO2 | Hydropyrolysis at 400 °C, 100 bar H2 for 4 h | 78% yield of biooil | [84] |
| 5 | Kraft lignin | NiMo/MgO-La2O3 | Hydropyrolysis at 350 °C, 100 bar H2 for 4 h | 26% yield of monomeric cyclic hydrocarbon | [15] |
| 6 | Kraft lignin | Limonite (FeO(OH)·nH2O) | Hydropyrolysis at 450 °C, 100 bar H2 for 4 h | 34% yield of aromatic hydrocarbons | [85] |
| 7 | Kraft lignin | 10NiMoP/AC | Hydropyrolysis at 400–500 °C, 100 bar H2 for 4 h | 71% yield of biooil | [61] |
| 8 | Kraft lignin | NiMoP/SiO2 | Hydropyrolysis at 400 °C, 100 bar H2 for 2 h | 68% yield of biooil, (52% sel. of monomeric compounds and 31% sel. of alkylphenols) | [2] |
| 9 | Pinewood | W2C/γ-Al2O3 | Hydropyrolysis at 500 °C, H2 ambience for 30 s | 17% BTX, 18% alkyl benzenes, 15–17% of naphthalenes | [87] |
| 10 | Lignin extracted from Chinese fir | NixMo/ZrO2 | Hydropyrolysis at 400 °C under atmospheric H2 | 18–19% yield of aromatics and 6% cycloalkanes | [88] |
| 11 | Industrial (Etek) lignin | Fe/ZrO2 | Hydropyrolysis at 400 °C under atmospheric H2 | 65–67% Sel. of non-oxygenated aromatics (BTX) | [89] |
| 12 | Stem wood | Pt/TiO2 | Hydropyrolysis at 600 °C under H2 | 35–37% yield of biooil | [90] |
| 13 | Kraft lignin | Y zeolite | Hydropyrolysis at 400 °C, 35 bar H2 for 5 h | 21% yield of biooil | [91] |
| 14 | Kraft lignin | NiMo/Y zeolite | Hydropyrolysis at 400 °C, 35 bar H2 for 5 h | 31% yield of biooil | [91] |
| 15 | Pine sawdust | NiMo/HZSM-5 | Hydropyrolysis at 320 °C and 50 bar H2 | 24 wt.% of yield w.r.t dried biomass | [92] |
6. Key Observations of Lignin Hydropyrolysis
- Hydropyrolysis is an industry-relevant process, it does not require solvent/medium which leads to low operation costs, and it is techno-economically viable.
- Generally, the non-catalytic pyrolysis resulted in oxygenated aromatic compounds including alkenyl-, aldo-, keto-, and carboxy-substituted compounds.
- In the acid-catalyzed hydropyrolysis of lignin, the HZSM-5 showed good activity for the biooil, which consists of a mixture of compounds such as phenols, alkyl-phenols, methoxyphenols, and non-oxygenated aromatics.
- The addition of base to acid-catalyzed lignin hydropyrolysis improves the cleavage of methoxy groups in the methoxyphenolic compounds and reveals a high amount of non-oxygenated compounds. Moreover, the additional base suppressed the condensation/polymerization (in between formed monomeric aromatics during acid-catalyzed hydropyrolysis) reaction and avoids the solid/char/polyaromatics formation.
- The Pd/C catalyst showed a high yield of biooil (81%), consisting of alkyl-cyclohexanones as major components. However, the Pd-containing bimetallic catalysts such as Pd-Fe and Pd-Co catalysts showed a high quantity of non-oxygenated aromatics, predominantly benzene and alkyl benzenes.
- The Ru-based catalysts showed a high yield (75–78%) of biooil, consisting of alkyl/hydroxyl phenols, non-oxygenated aromatic compounds (alkyl-substituted benzenes, benzene, toluene, xylenes, naphthalenes), and cycloalkanes.
- The Ni-containing bimetallic catalysts extensively reported for hydropyrolysis of lignin and the catalytic system’s synergetic effect play important roles. For example, in the Ni-Mo bimetallic catalytic system, the Mo metal initiates the cleavage of methoxy moieties in the methoxyphenolics, and the Ni metal promotes the cleavage of hydroxyl moieties in the phenolic compounds and also initiates the hydrogenation of the benzene ring. The combined properties of bimetallic catalyst provided a good yield of monomeric non-oxygen-containing aromatic/alicyclic hydrocarbons.
- The oxophilicity character of metal (for example, Mo and Fe) interacts with oxygenated aromatic compounds, which leads to the closer distance between the catalyst active sites and the oxygenated reactant. Thus, the strategy enhances the hydrodeoxygenation capacity of lignin-oxygenates.
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stummann, M.Z.; Elevera, E.; Hansen, A.B.; Hansen, L.P.; Beato, P.; Davidsen, B.; Wiwel, P.; Gabrielsen, J.; Jensen, P.A.; Jensen, A.D.; et al. Catalytic hydropyrolysis of biomass using supported CoMo catalysts–Effect of metal loading and support acidity. Fuel 2020, 264, 116807. [Google Scholar] [CrossRef]
- Osorio Velasco, J.; vander Linden, I.; Deuss, P.J.; Heeres, H.J. Efficient depolymerization of lignins to alkyl phenols using phosphided NiMo catalysts. Catal. Sci. Technol. 2021, 11, 5158–5170. [Google Scholar] [CrossRef]
- Ambursa, M.M.; Juan, J.C.; Yahaya, Y.; Taufiq-Yap, Y.H.; Lin, Y.-C.; Lee, H.V. A review on catalytic hydrodeoxygenation of lignin to transportation fuels by using nickel-based catalysts. Renew. Sustain. Energy Rev. 2021, 138, 110667. [Google Scholar] [CrossRef]
- Gundekari, S.; Srinivasan, K. Chemo- and Regioselective Synthesis of Arylated γ-Valerolactones from Bio-based Levulinic Acid with Aromatics Using H-β Zeolite Catalyst. ChemCatChem 2019, 11, 1102–1111. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Gundekari, S.; Mani, M.; Mitra, J.; Srinivasan, K. Selective preparation of renewable ketals from biomass-based carbonyl compounds with polyols using β-zeolite catalyst. Mol. Catal. 2022, 524, 112269. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Gundekari, S.; Mitra, J.; Varkolu, M. Chapter 4—Classification, characterization, and properties of edible and non-edible biomass feedstocks. In Advanced Functional Solid Catalysts for Biomass Valorization; Mustansar Hussain, C., Sudarsanam, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 89–120. [Google Scholar]
- Gundekari, S.; Mitra, J.; Bhaskar, T.; Srinivasan, K. Chapter 4—Preparation of cyclohexanol intermediates from lignin through catalytic intervention. In Biomass, Biofuels, Biochemicals; Bhaskar, T., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 57–82. [Google Scholar]
- Zou, Q.; Lin, W.; Xu, D.; Wu, S.; Mondal, A.K.; Huang, F. Study the effect of zeolite poresize and acidity on the catalytic pyrolysis of Kraftlignin. Fuel Process. Technol. 2022, 237, 107467. [Google Scholar] [CrossRef]
- Zhu, Y.; Song, W.; Yao, R.; Zhao, Y.; Xu, G. In-situ catalytic hydropyrolysis of lignin for the production of aromatic rich bio-oil. J. Energy Inst. 2022, 101, 187–193. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, J.H.; Park, J.; Kim, J.K.; An, D.; Song, I.K.; Choi, J.W. Catalytic pyrolysis of lignin over HZSM-5 catalysts: Effect of various parameters on the production of aromatic hydrocarbon. J. Anal. Appl. Pyrolysis 2015, 114, 273–280. [Google Scholar] [CrossRef]
- Pandey, M.P.; Kim, C.S. Lignin Depolymerization and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Singh, R.; Krishna, B.B.; Mishra, G.; Kumar, J.; Bhaskar, T. Strategies for selection of thermo-chemical processes for the valorisation of biomass. Renew. Energy 2016, 98, 226–237. [Google Scholar] [CrossRef]
- Kumar, C.R.; Anand, N.; Kloekhorst, A.; Cannilla, C.; Bonura, G.; Frusteri, F.; Barta, K.; Heeres, H.J. Solvent free depolymerization of Kraft lignin to alkyl-phenolics using supported NiMo and CoMo catalysts. Green Chem. 2015, 17, 4921–4930. [Google Scholar] [CrossRef]
- Verziu, M.; Tirsoaga, A.; Cojocaru, B.; Bucur, C.; Tudora, B.; Richel, A.; Aguedo, M.; Samikannu, A.; Mikkola, J.P. Hydrogenolysis of lignin over Ru-based catalysts: The role of the ruthenium in a ligninfragmentation process. Mol. Catal. 2018, 450, 65–76. [Google Scholar] [CrossRef]
- Wang, H.; Male, J.; Wang, Y. Recent Advances in Hydrotreating of Pyrolysis Bio-Oil and Its Oxygen-Containing Model Compounds. ACS Catal. 2013, 3, 1047–1070. [Google Scholar] [CrossRef]
- Xie, W.; Liang, J.; Morgan, H.M.; Zhang, X.; Wang, K.; Mao, H.; Bu, Q. Ex-situ catalytic microwave pyrolysis of lignin over Co/ZSM-5 to upgrade bio-oil. J. Anal. Appl. Pyrolysis 2018, 132, 163–170. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Zacher, A.H.; Wang, L.; Ren, S.; Liang, J.; Wei, Y.; Liu, Y.; Tang, J.; Zhang, Q.; et al. A review of catalytic hydrodeoxygenation of lignin-derived phenols from biomass pyrolysis. Bioresour. Technol. 2012, 124, 470–477. [Google Scholar] [CrossRef]
- Li, P.; Shi, X.; Jiang, L.; Wang, X.; Song, J.; Fang, S.; Bai, J.; Chang, C.; Pang, S. Synergistic enhancement of bio-oil quality through hydrochloricor acetic acid- washing pretreatment and catalytic fast pyrolysis of biomass. Ind. Crop. Prod. 2022, 187, 115474. [Google Scholar] [CrossRef]
- Zhou, N.; Thilakarathna, W.P.D.W.; He, Q.S.; Rupasinghe, H.P.V. A Review: Depolymerization of Lignin to Generate High-Value Bio-Products: Opportunities, Challenges, and Prospects. Front. Energy Res. 2022, 9, 758744. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; deSanti, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef]
- Gundekari, S.; Karmee, S.K. Selective Synthesis of Cyclohexanol Intermediates from Lignin-Based Phenolics and Diaryl Ethers using Hydrogen over Supported Metal Catalysts: A Critical Review. Catal. Surv. Asia 2021, 25, 1–26. [Google Scholar] [CrossRef]
- Roy, R.; Rahman, M.S.; Amit, T.A.; Jadhav, B. Recent Advances in Lignin Depolymerization Techniques: A Comparative Overview of Traditional and Greener Approaches. Biomass 2022, 2, 130–154. [Google Scholar] [CrossRef]
- Garcia, A.C.; Cheng, S.; Cross, J.S. Solvolysis of Kraft Lignin to Bio-Oil: A Critical Review. Clean Technol. 2020, 2, 513–528. [Google Scholar] [CrossRef]
- Romanenko, I.; Kurz, F.; Baumgarten, R.; Jevtovikj, I.; Lindner, J.-P.; Kundu, A.; Kindler, A.; Schunk, S.A. Lignin Depolymerization in the Presence of Base, Hydrogenation Catalysts, and Ethanol. Catalysts 2022, 12, 158. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Liakakou, E.T.; Vreugdenhil, B.J.; Cerone, N.; Zimbardi, F.; Pinto, F.; André, R.; Marques, P.; Mata, R.; Girio, F. Gasification of lignin-rich residues for the production of biofuels via syn gas fermentation: Comparison of gasification technologies. Fuel 2019, 251, 580–592. [Google Scholar] [CrossRef]
- Roman-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethyl furan for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.B.; Kalam, M.A.; Zhang, Z.; Masjuki, H.H. Sustainable production of furan-based oxygenated fuel additives from pentose-rich biomass residues. Energy Convers. Manag. X 2022, 14, 100222. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Liu, R.; Wang, S.; Chen, L.; Li, K. Hydrodeoxygenation of Lignin-Derived Phenolic Monomers and Dimers to Alkane Fuels over Bi functional Zeolite-Supported Metal Catalysts. Acs Sustain. Chem. Eng. 2014, 2, 683–691. [Google Scholar] [CrossRef]
- Herreros, J.M.; Jones, A.; Sukjit, E.; Tsolakis, A. Blending lignin-derived oxygenate in enhanced multi-component diesel fuel for improved emissions. Appl. Energy 2014, 116, 58–65. [Google Scholar] [CrossRef]
- RajeshKumar, B.; Saravanan, S.; NiranjanKumar, R.; Nishanth, B.; Rana, D.; Nagendran, A. Effect of lignin-derived cyclohexanol on combustion, performance and emissions of a direct-injection agricultural diesel engine under naturally aspirated and exhaust gas recirculation (EGR) modes. Fuel 2016, 181, 630–642. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Zhu, L.; Wu, J.; Chen, S. From lignocellulosic biomass to renewable cycloalkanes for jet fuels. Green Chem. 2015, 17, 4736–4747. [Google Scholar] [CrossRef]
- Kloekhorst, A.; Wildschut, J.; Heeres, H.J. Catalytic hydro treatment of pyrolytic lignins to give alkylphenolics and aromatics using a supported Ru catalyst. Catal. Sci. Technol. 2014, 4, 2367–2377. [Google Scholar] [CrossRef]
- Stone, M.L.; Webber, M.S.; Mounfield, W.P.; Bell, D.C.; Christensen, E.; Morais, A.R.C.; Li, Y.; Anderson, E.M.; Heyne, J.S.; Beckham, G.T.; et al. Continuous hydrodeoxygenation of lignin to jet-range aromatic hydrocarbons. Joule 2022, 6, 2324–2337. [Google Scholar] [CrossRef]
- Talibi, M.; Hellier, P.; Ladommatos, N. Impact of increasing methyl branches in aromatic hydrocarbons on diesel engine combustion and emissions. Fuel 2018, 216, 579–588. [Google Scholar] [CrossRef]
- Bi, P.; Wang, J.; Zhang, Y.; Jiang, P.; Wu, X.; Liu, J.; Xue, H.; Wang, T.; Li, Q. From lignin to cycloparaffins and aromatics:Directional synthesis of jet and diesel fuel range biofuels using biomass. Bioresour. Technol. 2015, 183, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Kundra, M.; Grall, T.; Ng, D.; Xie, Z.; Hornung, C.H. Continuous Flow Hydrogenation of Flavorings and Fragrances Using 3D-Printed Catalytic Static Mixers. Ind. Eng. Chem. Res. 2021, 60, 1989–2002. [Google Scholar] [CrossRef]
- Sreenavya, A.; Sahu, A.; Sakthivel, A. Hydrogenation of Lignin-Derived Phenolic Compound Eugenol over Ruthenium-Containing Nickel Hydrotalcite-Type Materials. Ind. Eng. Chem. Res. 2020, 59, 11979–11990. [Google Scholar] [CrossRef]
- Chen, G.; Liu, J.; Li, X.; Zhang, J.; Yin, H.; Su, Z. Investigation on catalytic hydrodeoxygenation of eugenol blend with light fraction in bio-oil over Ni-based catalysts. Renew. Energy 2020, 157, 456–465. [Google Scholar] [CrossRef]
- Guan, W.; Chen, X.; Tsang, C.W.; Hu, H.; Liang, C. Highly Dispersed Rh/NbOx Invoking High Catalytic Performances for the Valorization of Lignin Monophenols and Lignin Oil into Aromatics. ACS Sustain. Chem. Eng. 2021, 9, 3529–3541. [Google Scholar] [CrossRef]
- Xu, L.; Han, Z.; Zhang, Y.; Fu, Y. Insitu synthesis of molybdenumoxide@N-doped carbon from biomass for selective vaporphase hydrodeoxygenation of lignin-derived phenols under H2 atmosphere. RSC Adv. 2016, 6, 108217–108228. [Google Scholar] [CrossRef]
- Nimmanwudipong, T.; Runnebaum, R.C.; Ebeler, S.E.; Block, D.E.; Gates, B.C. Upgrading of Lignin-Derived Compounds: Reactions of Eugenol Catalyzed by HYZeolite and by Pt/γ-Al2O3. Catal. Lett. 2012, 142, 151–160. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Yu, M.J.; Ko, C.H.; Jeon, J.-K.; Jae, J.; Park, S.H.; Jung, S.-C.; Park, Y.-K. Catalytic Hydrodeoxygenation of Bio-oil Model Compounds over Pt/HY Catalyst. Sci. Rep. 2016, 6, 28765. [Google Scholar] [CrossRef]
- Lu, X.; Guo, H.; Wang, D.; Xiu, P.; Qin, Y.; Chen, J.; Xu, C.; Gu, X. A review on catalytic conversion of ligninin to high-value chemicals over Ni-based catalysts. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Deepa, A.K.; Dhepe, P.L. Function of Metals and Supports on the Hydrodeoxygenation of Phenolic Compounds. ChemPlusChem 2014, 79, 1573–1583. [Google Scholar] [CrossRef]
- Liu, X.; Jia, W.; Xu, G.; Zhang, Y.; Fu, Y. Selective Hydrodeoxygenation of Lignin-Derived Phenols to Cyclohexanols over Co-Based Catalysts. ACS Sustain. Chem. Eng. 2017, 5, 8594–8601. [Google Scholar] [CrossRef]
- Gundekari, S.; Biswas, B.; Bhaskar, T.; Srinivasan, K. Preparation of cyclohexanol from lignin-based phenolic concoction using controlled hydrogen delivery tool over in-situ Ru catalyst. Biomass Bioenergy 2022, 161, 106448. [Google Scholar] [CrossRef]
- Xu, G.-Y.; Guo, J.-H.; Qu, Y.-C.; Zhang, Y.; Fu, Y.; Guo, Q.-X. Selective hydrodeoxygenation of lignin-derived phenols to alkylcyclohexanols over a Ru-solid base bifunctional catalyst. Green Chem. 2016, 18, 5510–5517. [Google Scholar] [CrossRef]
- Qiu, S.; Xu, Y.; Weng, Y.; Ma, L.; Wang, T. Efficient Hydrogenolysis of Guaiacol over Highly Dispersed Ni/MCM-41 Catalyst Combined with HZSM-5. Catalysts 2016, 6, 134. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.-Y.; Huang, Y.-B.; Pang, H.; Liu, X.-X.; Fu, Y. Hydrodeoxygenation of lignin-derived phenols into alkanes over carbon nanotube supported Ru catalysts in biphasic systems. Green Chem. 2015, 17, 1710–1717. [Google Scholar] [CrossRef]
- Piegsa, A.; Korth, W.; Demir, F.; Jess, A. Hydrogenation and Ring Opening of Aromatic and Naphthenic Hydrocarbons over Noble Metal (Ir, Pt, Rh)/Al2O3 Catalysts. Catal. Lett. 2012, 142, 531–540. [Google Scholar] [CrossRef]
- Cheah, Y.W.; Salam, M.A.; Arora, P.; Öhrman, O.; Creaser, D.; Olsson, L. Role of transition metals on MoS2-based supported catalysts for hydrodeoxygenation (HDO) of propylguaiacol. Sustain. Energy Fuels 2021, 5, 2097–2113. [Google Scholar] [CrossRef]
- Prabhudesai, V.S.; Gurrala, L.; Vinu, R. Catalytic Hydrodeoxygenation of Lignin-Derived Oxygenates: Catalysis, Mechanism, and Effect of Process Conditions. Energy Fuels 2022, 36, 1155–1188. [Google Scholar] [CrossRef]
- Campos Fraga, M.M.; Lacerdade Oliveira Campos, B.; Hendrawidjaja, H.; CarrielSchmitt, C.; Raffelt, K.; Dahmen, N. Fast Pyrolysis Oil Upgrading via HDO with Fe-Promoted Nb2O5-Supported Pd-Based Catalysts. Energies 2022, 15, 4762. [Google Scholar] [CrossRef]
- Martinez-Klimov, M.; Mäki-Arvela, P.; Çiftçi, A.; Kumar, N.; Eränen, K.; Peurla, M.; Hensen, E.J.M.; Murzin, D.Y. Bifunctional Pt–Re Catalysts in Hydrodeoxygenation of Isoeugenol as a Model Compound for Renewable Jet Fuel Production. ACS Eng. Au 2022, 2, 436–449. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.; He, J.; Kumar, R.; Lu, Q. Catalytic pyrolysis of lignocellulosic biomass: A review of variations in process factors and system structure. Renew. Sustain. Energy Rev. 2020, 134, 110305. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; De Oliveira Matias, J.C.; Da Silva Catalão, J.P. Chapter11-Applications for Torrefied Biomass. In Torrefaction of Biomass for Energy Applications; Nunes, L.J.R., De Oliveira Matias, J.C., Da Silva Catalão, J.P., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 203–214. [Google Scholar]
- Tran, N.T.T.; Uemura, Y.; Ramli, A.; Trinh, T.H. Vapor-phase hydrodeoxygenation of lignin-derived bio-oil over Al-MCM-41 supported Pd-Co and Pd-Fe catalysts. Mol. Catal. 2022, 523, 111435. [Google Scholar] [CrossRef]
- Chowdari, R.K.; Agarwal, S.; Heeres, H.J. Hydrotreatment of Kraft Lignin to Alkyl phenolics and Aromatics Using Ni, Mo, and W Phosphides Supported on Activated Carbon. ACS Sustain. Chem. Eng. 2019, 7, 2044–2055. [Google Scholar] [CrossRef] [Green Version]
- Mäki-Arvela, P.; Murzin, D.Y. Hydrodeoxygenation of Lignin-Derived Phenols: From Fundamental Studies towards Industrial Applications. Catalysts 2017, 7, 265. [Google Scholar] [CrossRef] [Green Version]
- Héroguel, F.; Nguyen, X.T.; Luterbacher, J.S. Catalyst Support and Solvent Effects during Lignin Depolymerization and Hydrodeoxygenation. ACS Sustain. Chem. Eng. 2019, 7, 16952–16958. [Google Scholar] [CrossRef]
- Zheng, N.; Zhang, J.; Wang, J. Parametric study of two-stage hydropyrolysis of lignocellulosic biomass for production of gaseous and light aromatic hydrocarbons. Bioresour. Technol. 2017, 244, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Güell, A.J.; Li, C.Z.; Herod, A.A.; Stokes, B.J.; Hancock, P.; Kandiyot, R. Effect of H2-pressure on the structures of bio-oils from the mild hydropyrolysis of biomass. Biomass Bioenergy 1993, 5, 155–171. [Google Scholar] [CrossRef]
- Dash, S.; Thakur, S.; Bhavanam, A.; Gera, P. Catalytic pyrolysis of alkaline lignin:A systematic kinetic study. Bioresour. Technol. Rep. 2022, 18, 101064. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, Z.; Yang, Q.; Ben, H.; Seufitelli, G.V.S.; Resende, F.L.P. Parametric study of catalytic hydropyrolysis of rice husk over a hierarchical micro-mesoporous composite catalyst for production of light alkanes, alkenes, and liquid aromatic hydrocarbons. Fuel 2022, 310, 122457. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, Y.-M.; Jae, J.; Sung, B.H.; Jung, S.-C.; Kim, S.C.; Jeon, J.-K.; Park, Y.-K. Catalytic pyrolysis of lignin using a two-stage fixed bed reactor comprised of in-situ natural zeolite and ex-situ HZSM-5. J. Anal. Appl. Pyrolysis 2016, 122, 282–288. [Google Scholar] [CrossRef]
- Biswas, B.; Pandey, N.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Pyrolysis of agricultural biomass residues: Comparative study of corncob, wheatstraw, rice straw and ricehusk. Bioresour. Technol. 2017, 237, 57–63. [Google Scholar] [CrossRef]
- Li, W.; Zhou, S.; Xue, Y.; Lee, Y.-J.; Smith, R.; Bai, X. Understanding Low-Pressure Hydropyrolysis of Lignin Using Deuterated Sodium Formate. ACS Sustain. Chem. Eng. 2017, 5, 8939–8950. [Google Scholar] [CrossRef]
- Wang, H.; Li, T.; Su, J.; Miao, K.; Wang, K. Noncatalytic hydropyrolysis of lignin in a high pressure micro-pyrolyzer. Fuel Process. Technol. 2022, 233, 107289. [Google Scholar] [CrossRef]
- Pienihäkkinen, E.; Lindfors, C.; Ohra-aho, T.; Lehtonen, J.; Granström, T.; Yamamoto, M.; Oasmaa, A. Fast Pyrolysis of Hydrolysis Lignin in Fluidized Bed Reactors. Energy Fuels 2021, 35, 14758–14769. [Google Scholar] [CrossRef]
- Jiang, W.; Cao, J.-P.; Yang, Z.; Xie, J.-X.; Zhao, L.; Zhu, C.; Zhang, C.; Zhao, X.-Y.; Zhao, Y.-P.; Zhang, J.-L. Hydrodeoxygenation of lignin and its model compounds to hydrocarbon fuels over a bifunctional Ga-doped HZSM-5 supported metal Ru catalyst. Appl. Catal. A: Gen. 2022, 633, 118516. [Google Scholar] [CrossRef]
- Balagurumurthy, B.; Bhaskar, T. Hydropyrolysis of lignocellulosic biomass: State of the art review. Biomass Convers. Biorefinery 2014, 4, 67–75. [Google Scholar] [CrossRef]
- Santana, J.A.; Carvalho, W.S.; Ataíde, C.H. Catalytic effect of ZSM-5 zeolite and HY-340 niobic acid on the pyrolysis of industrial kraftlignins. Ind. Crop. Prod. 2018, 111, 126–132. [Google Scholar] [CrossRef]
- SantanaJunior, J.A.; Menezes, A.L.; Ataíde, C.H. Catalytic upgrading of fast hydropyrolysis vapors from industrial Kraftlignins using ZSM-5 zeolite and HY-340 niobic acid. J. Anal. Appl. Pyrolysis 2019, 144, 104720. [Google Scholar] [CrossRef]
- Sirous-Rezaei, P.; Park, Y.-K. Catalytic hydropyrolysis of lignin: Suppression of coke formation in mild hydrodeoxygenation of lignin-derived phenolics. Chem. Eng. J. 2020, 386, 121348. [Google Scholar] [CrossRef]
- Spivey, J.J.; Hutchings, G. Catalytic aromatization of methane. Chem. Soc. Rev. 2014, 43, 792–803. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Ishikawa, M.; Tamura, M.; Tomishige, K. Selective production of cyclohexanol and methanol from guaiacol over Ru catalyst combined with MgO. Green Chem. 2014, 16, 2197–2203. [Google Scholar] [CrossRef]
- Wu, J.; Chang, G.; Li, X.; Li, J.; Guo, Q. Effects of NaOH on the catalytic pyrolysis of lignin/HZSM-5 to prepare aromatic hydrocarbons. J. Anal. Appl. Pyrolysis 2020, 146, 104775. [Google Scholar] [CrossRef]
- Meier, D.; Ante, R.; Faix, O. Catalytic hydropyrolysis of lignin:Influence of reaction conditions on the formation and composition of liquid products. Bioresour. Technol. 1992, 40, 171–177. [Google Scholar] [CrossRef]
- Meier, D.; Berns, J.; Faix, O. High Liquid Yields from Lignin Via Catalytic Hydropyrolysls. In Advances in Thermochemical Biomass Conversion; Bridgwater, A.V., Ed.; Springer: Dordrecht, The Netherlands, 1993; pp. 1016–1031. [Google Scholar]
- Jan, O.; Marchand, R.; Anjos, L.C.A.; Seufitelli, G.V.S.; Nikolla, E.; Resende, F.L.P. Hydropyrolysis of Lignin Using Pd/HZSM-5. Energy Fuels 2015, 29, 1793–1800. [Google Scholar] [CrossRef] [Green Version]
- Kloekhorst, A.; Heeres, H.J. Catalytic Hydrotreatmentof AlcellLigninUsing Supported Ru, Pd, and Cu Catalysts. Acs Sustain. Chem. Eng. 2015, 3, 1905–1914. [Google Scholar] [CrossRef]
- Agarwal, S.; Chowdari, R.K.; Hita, I.; Heeres, H.J. Experimental Studies on the Hydrotreatment of Kraft Lignin to Aromatics and Alkylphenolics Using Economically Viable Fe-Based Catalysts. ACS Sustain. Chem. Eng. 2017, 5, 2668–2678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- deWild, P.J.; Huijgen, W.J.J.; Kloekhorst, A.; Chowdari, R.K.; Heeres, H.J. Biobased alkyl phenols from lignins via a two-step pyrolysis–Hydrodeoxygenation approach. Bioresour. Technol. 2017, 229, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, K.; He, S.; Seshan, K.; Selvam, P.; Vinu, R. Selective production of aromatic hydrocarbons from lignocellulosic biomass via catalytic fast-hydropyrolysis using W2C/γ-Al2O3. Catal. Commun. 2018, 110, 68–73. [Google Scholar] [CrossRef]
- Li, T.; Su, J.; Wang, H.; Wang, C.; Xie, W.; Wang, K. Catalytic hydropyrolysis of lignin using NiMo-doped catalysts:Catalyst evaluation and mechanism analysis. Appl. Energy 2022, 316, 119115. [Google Scholar] [CrossRef]
- Lonchay, W.; Bagnato, G.; Sanna, A. Highly selective hydropyrolysis of lignin waste to benzene, toluene and xylene in presence of zirconia supported ironc atalyst. Bioresour. Technol. 2022, 361, 127727. [Google Scholar] [CrossRef] [PubMed]
- Shafaghat, H.; Linderberg, M.; Janosik, T.; Hedberg, M.; Wiinikka, H.; Sandström, L.; Johansson, A.-C. Enhanced Biofuel Production via Catalytic Hydropyrolysis and Hydro-Coprocessing. Energy Fuels 2022, 36, 450–462. [Google Scholar] [CrossRef]
- AbdusSalam, M.; WayneCheah, Y.; HoangHo, P.; Bernin, D.; Achour, A.; Nejadmoghadam, E.; Öhrman, O.; Arora, P.; Olsson, L.; Creaser, D. Elucidating the role of NiMoS-USY during the hydrotreatment of Kraft lignin. Chem. Eng. J. 2022, 442, 136216. [Google Scholar] [CrossRef]
- Shi, J.; Sun, L.; Yan, H.; Wang, J. Catalytic Hydrotreatment of Pine Sawdust Hydropyrolysis Vapor over Ni, Mo-Impregnated HZSM-5 for Optimal Production of Gasoline Components. Energy Fuels 2022, 36, 932–944. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gundekari, S.; Karmee, S.K. Catalytic Hydropyrolysis of Lignin for the Preparation of Cyclic Hydrocarbon-Based Biofuels. Catalysts 2022, 12, 1651. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12121651
Gundekari S, Karmee SK. Catalytic Hydropyrolysis of Lignin for the Preparation of Cyclic Hydrocarbon-Based Biofuels. Catalysts. 2022; 12(12):1651. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12121651
Chicago/Turabian StyleGundekari, Sreedhar, and Sanjib Kumar Karmee. 2022. "Catalytic Hydropyrolysis of Lignin for the Preparation of Cyclic Hydrocarbon-Based Biofuels" Catalysts 12, no. 12: 1651. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12121651