Assessment of a Novel Photocatalytic TiO2-Zirconia Ultrafiltration Membrane and Combination with Solar Photo-Fenton Tertiary Treatment of Urban Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Analytical Determinations
2.3. Experimental Setup
2.3.1. Solar Photo-Fenton Treatment
2.3.2. Retention of P. Aeruginosa
3. Results and Discussion
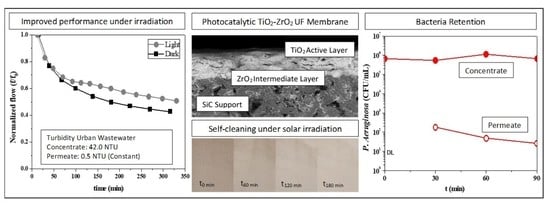
3.1. Fouling and Self-Cleaning Properties
3.2. Solar Photo-Fenton Treatment of Membrane Streams
3.3. Retention of P. Aeruginosa
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| AOPs | Advanced oxidation processes |
| CAF | Caffeine |
| CBZ | Carbamazepine |
| CF | Concentration factor |
| CPC | Compound parabolic collector |
| DCF | Diclofenac |
| EDDS | Ethylenediamine-N: N′-disuccinic acid |
| EDX | Energy-dispersive X-ray spectroscopy |
| EPS | Extracellular polymeric substances |
| IC | Inorganic carbon |
| IMI | Imidacloprid |
| LOQ | Limit of quantification |
| MCs | micro contaminants |
| NF | Nanofiltration |
| PSH | Photo-induced super-hydrophilicity |
| RO | Reverse osmosis |
| SEM | Scanning electron microscopy |
| THI | hiacloprid |
| TOC | Total organic carbon |
| TSS | Total suspended solids |
| UF | Ultrafiltration |
| UWW | Urban wastewater |
| UWWTP | Urban wastewater treatment plant |
References
- Messaoudi, M.; Douma, M.; Tijani, N.; Messaoudi, L. Study of the permeability of tubular mineral membranes: Application to wastewater treatment. Heliyon 2021, 7, e06837. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Y.; Dudek, M.; Qin, J.; Gisle, Ø.; Stein, W.Ø. A multivariate study of backpulsing for membrane fouling mitigation in produced water treatment. J. Environ. Chem. Eng. 2021, 9, 104839. [Google Scholar] [CrossRef]
- Alawad, S.M.; Khalifa, A.E. Case Studies in Thermal Engineering Development of an efficient compact multistage membrane distillation module for water desalination. Case Stud. Therm. Eng. 2021, 25, 100979. [Google Scholar] [CrossRef]
- Comas, J.; Corominas, L. Balancing environmental quality standards and infrastructure upgrade costs for the reduction of microcontaminant loads in rivers. Wat. Res. 2018, 143, 632–641. [Google Scholar] [CrossRef]
- de Santiago-martín, A.; Meffe, R.; Teijón, G.; Martínez, V.; López-heras, I.; Alonso, C.; Arenas, M.; de Bustamante, I. Pharmaceuticals and trace metals in the surface water used for crop irrigation: Risk to health or natural attenuation? Sci. Total Environ. 2020, 705, 135825. [Google Scholar] [CrossRef]
- Asghar, A.; Lutze, H.V.; Tuerk, J.; Schmidt, T.C. Influence of water matrix on the degradation of organic micropollutants by ozone based processes: A review on oxidant scavenging mechanism. J. Hazard. Mater. 2022, 429, 128189. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Nunes, M.I. Recent trends and developments in Fenton processes for industrial wastewater treatment—A critical review. Environ. Res. 2021, 197, 110957. [Google Scholar] [CrossRef]
- Du, X.; Oturan, M.A.; Zhou, M.; Belkessa, N.; Su, P.; Cai, J.; Trellu, C.; Mousset, E. Nanostructured electrodes for electrocatalytic advanced oxidation processes: From materials preparation to mechanisms understanding and wastewater treatment applications. Appl. Catal. B Environ. 2021, 296, 120332. [Google Scholar] [CrossRef]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Ribeiro, A.R.L.; et al. Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef]
- Bouzerara, F.; Guvenc, C.M.; Demir, M.M. Fabrication and properties of novel porous ceramic membrane supports from the (Sig) diatomite and alumina mixtures. Bol. Soc. Esp. Cerám. Vidr. 2021; 1–10, in press. [Google Scholar] [CrossRef]
- Zsirai, T.; Qiblawey, H.; Ahmed, A.; Bach, S.; Watson, S.; Judd, S. Ceramic membrane filtration of produced water: Impact of membrane module. Sep. Purif. Technol. 2016, 165, 214–221. [Google Scholar] [CrossRef] [Green Version]
- da Silva Biron, D.; Dos Santos, V.; Zeni, M. Ceramic Membranes Applied in Separation Processes; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Gitis, V.; Rothenberg, G. Ceramic Membranes: Ceramic Membranes: New Opportunities and Practical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, L.; Graham, N.; Yu, W. Unraveling membrane fouling induced by chlorinated water versus surface water: Biofouling properties and microbiological investigation. Engineering, 2021; in press. [Google Scholar] [CrossRef]
- Hoslett, J.; Maria, T.; Malamis, S.; Ahmad, D.; van den Boogaert, I.; Katsou, E.; Ahmad, B.; Ghazal, H.; Simons, S.; Wrobel, L.; et al. Science of the Total Environment Surface water fi ltration using granular media and membranes: A review. Sci. Total Environ. 2018, 639, 1268–1282. [Google Scholar] [CrossRef] [PubMed]
- Czuba, K.; Bastrzyk, A.; Rogowska, A.; Janiak, K.; Pacyna, K.; Kossi, N.; Podstawczyk, D. Towards the circular economy—A pilot-scale membrane technology for the recovery of water and nutrients from secondary effluent. Sci. Total Environ. 2021, 791, 148266. [Google Scholar] [CrossRef] [PubMed]
- Kolesnyk, I.; Kujawa, J.; Bubela, H.; Konovalova, V.; Burban, A. Separation and Purification Technology Photocatalytic properties of PVDF membranes modi fi ed with g-C3N4 in the process of Rhodamines decomposition. Sep. Purif. Technol. 2020, 250, 117231. [Google Scholar] [CrossRef]
- Della-Flora, A.; Wilde, M.L.; Thue, P.S.; Lima, D.; Lima, E.C.; Sirtori, C. Combination of solar photo-Fenton and adsorption process for removal of the anticancer drug Flutamide and its transformation products from hospital wastewater. J. Hazard. Mater. 2020, 396, 122699. [Google Scholar] [CrossRef]
- Foteinis, S.; Monteagudo, J.M.; Durán, A.; Chatzisymeon, E. Environmental sustainability of the solar photo-Fenton process for wastewater treatment and pharmaceuticals mineralization at semi-industrial scale. Sci. Total Environ. 2018, 612, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Cheng, H. Chemical kinetic modeling of organic pollutant degradation in Fenton and solar photo-Fenton processes. J. Taiwan Inst. Chem. Eng. 2021, 123, 175–184. [Google Scholar] [CrossRef]
- Norén, A.; Fedje, K.K.; Strömvall, A.-M.; Rauch, S.; Andersson-Sköld, Y. Low impact leaching agents as remediation media for organotin and metal contaminated sediments. J. Environ. Manag. 2021, 282, 111906. [Google Scholar] [CrossRef]
- Vandevivere, P.C.; Saveyn, H.; Verstraete, W.; Feijtel, T.C.J.; Schowanek, D.R. Biodegradation of metal-[S,S]-EDDS complexes. Environ. Sci. Technol. 2001, 35, 1765–1770. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; di Somma, I.; Marotta, R.; Andreozzi, R. Homogeneous photo-Fenton processes at near neutral pH: A review. Appl. Catal. B Environ. 2017, 209, 358–371. [Google Scholar] [CrossRef]
- Vineyard, D.; Hicks, A.; Karthikeyan, K.G.; Barak, P. Economic analysis of electrodialysis, denitri fi cation, and anammox for nitrogen removal in municipal wastewater treatment. J. Clean. Prod. 2020, 262, 121145. [Google Scholar] [CrossRef]
- Igere, B.E.; Okoh, A.I.; Nwodo, U.U. Wastewater treatment plants and release: The vase of Odin for emerging bacterial contaminants, resistance and determinant of environmental wellness. Emerg. Contam. 2020, 6, 212–224. [Google Scholar] [CrossRef]
- del Mar Cendra, M.; Torrents, E. Pseudomonas aeruginosa biofilms and their partners in crime. Biotechnol. Adv. 2021, 49, 107734. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Gernjak, W.; Krzeminski, P.; Malato, S.; McArdell, C.S.; Perez, J.A.S.; Schaar, H.; Fatta-Kassinos, D. Best available technologies and treatment trains to address current challenges in urban wastewater reuse for irrigation of crops in EU countries. Sci. Total Environ. 2020, 710, 136312. [Google Scholar] [CrossRef] [PubMed]
- Coelho, F.E.B.; Deemter, D.; Candelario, V.M.; Boffa, V.; Malato, S.; Magnacca, G. Development of a Photocatalytic Zirconia-Titania Ultrafiltration Membrane with Anti-fouling and Self-cleaning Properties. J. Environ. Chem. Eng. 2021, 9, 106671. [Google Scholar] [CrossRef]
- Federal Office for the Environment FOEN Water Division. Reporting for Switzerland under the Protocol on Water and Health. 2019. Available online: https://unece.org/DAM/env/water/Protocol_reports/reports_pdf_web/Switzerland_summary_report_en.pdf (accessed on 12 May 2022).
- Hosseinkhan, N.; Allahverdi, A.; Abdolmaleki, F. The novel potential multidrug-resistance biomarkers for Pseudomonas aeruginosa lung infections using transcriptomics data analysis. Informatics Med. Unlocked. 2021, 22, 100509. [Google Scholar] [CrossRef]
- Horna, G.; Ruiz, J. Type 3 secretion system of Pseudomonas aeruginosa. Microbiol. Res. 2021, 246, 126719. [Google Scholar] [CrossRef]
- Shaker, M.M.; Al-Hadrawi, H.A.N. Measuring the effectiveness of antibiotics against Pseudomonas aeruginosa and Escherichia coli that isolated from urinary tract infection patients in Al-Najaf city in Iraq. Mater. Today Proc. in press 2021, 10–13. [Google Scholar] [CrossRef]
- Liang, C.; Huang, C.F.; Mohanty, N.; Kurakalva, R.M. A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere 2008, 73, 1540–1543. [Google Scholar] [CrossRef]
- Lozano, C.; López, M.; Rojo-Bezares, B.; Sáenz, Y. Antimicrobial susceptibility testing in pseudomonas aeruginosa biofilms: One step closer to a standardized method. Antibiotics 2020, 9, 880. [Google Scholar] [CrossRef]
- Díez-Aguilar, M.; Martínez-García, L.; Cantón, R.; Morosini, M.I. Is a new standard needed for diffusion methods for in vitro susceptibility testing of fosfomycin against Pseudomonas aeruginosa? Antimicrob. Agents Chemother. 2016, 60, 1158–1161. [Google Scholar] [CrossRef] [Green Version]
- Mizunaga, S.; Kamiyama, T.; Fukuda, Y.; Takahata, M.; Mitsuyama, J. Influence of inoculum size of Staphylococcus aureus and Pseudomonas aeruginosa on in vitro activities and in vivo efficacy of fluoroquinolones and carbapenems. J. Antimicrob. Chemother. 2005, 56, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.M.; In, I.; Park, S.Y. Study of photo-induced hydrophilicity and self-cleaning property of glass surfaces immobilized with TiO2 nanoparticles using catechol chemistry. Surf. Coatings Technol. 2016, 294, 75–82. [Google Scholar] [CrossRef]
- Saini, A.; Arora, I.; Ratan, J.K. Photo-induced hydrophilicity of microsized-TiO2 based self-cleaning cement. Mater. Lett. 2020, 260, 26888. [Google Scholar] [CrossRef]
- Rani, C.N.; Karthikeyan, S.; Arockia, S.P. Photocatalytic ultrafiltration membrane reactors in water and wastewater treatment—A review. Chem. Eng. Process. Process Intensif. 2021, 165, 108445. [Google Scholar] [CrossRef]
- Deemter, D.; Oller, I.; Amat, A.M.; Malato, S. Effect of salinity on preconcentration of contaminants of emerging concern by nanofiltration: Application of solar photo-Fenton as a tertiary treatment. Sci. Total Environ. 2020, 756, 143593. [Google Scholar] [CrossRef]
- Rommozzi, E.; Giannakis, S.; Giovannetti, R.; Vione, D.; Pulgarin, C. Detrimental vs. beneficial influence of ions during solar (SODIS) and photo-Fenton disinfection of E. coli in water: (Bi)carbonate, chloride, nitrate and nitrite effects. Appl. Catal. B Environ. 2020, 270, 118877. [Google Scholar] [CrossRef]
- Gonçalves, B.R.; Guimarães, R.O.; Batista, L.L.; Ueira-Vieira, C.; Starling, M.C.V.M.; Trovó, A.G. Reducing toxicity and antimicrobial activity of a pesticide mixture via photo-Fenton in different aqueous matrices using iron complexes. Sci. Total Environ. 2020, 740, 140152. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Molina, P.; Sánchez, J.L.G.; Malato, S.; Pérez-Estrada, L.A.; Pérez, J.A.S. Effect of volumetric rate of photon absorption on the kinetics of micropollutant removal by solar photo-Fenton with Fe3+-EDDS at neutral pH. Chem. Eng. J. 2018, 331, 84–92. [Google Scholar] [CrossRef]
- Li, J.; Mailhot, G.; Wu, F.; Deng, N. Journal of Photochemistry and Photobiology A: Chemistry Photochemical efficiency of Fe (III)-EDDS complex: OH radical production. J. Photochem. Photobiol. A Chem. 2010, 212, 1–7. [Google Scholar] [CrossRef]
- Kanafin, Y.N.; Makhatova, A.; Zarikas, V.; Arkhangelsky, E.; Poulopoulos, S.G. Photo-Fenton-Like Treatment of Municipal Wastewater. Catalysts 2021, 11, 1206. [Google Scholar] [CrossRef]
- Iglewski, B. Pseudomonas. In Medical Microbiology, 4th ed.; Baron, S., Ed.; The University of Texas: Galveston, TX, USA, 1996. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/books/NBK8326/%0A (accessed on 12 May 2022).
- Formosa, C.; Grare, M.; Duval, R.E.; Dague, E. Nanoscale effects of antibiotics on P. aeruginosa. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Harimawan, A.; Ting, Y. Colloids and Surfaces B: Biointerfaces Investigation of extracellular polymeric substances (EPS) properties of P. aeruginosa and B. subtilis and their role in bacterial adhesion. Colloids Surf. B Biointerfaces 2016, 146, 459–467. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deemter, D.; Coelho, F.E.B.; Oller, I.; Malato, S.; Amat, A.M. Assessment of a Novel Photocatalytic TiO2-Zirconia Ultrafiltration Membrane and Combination with Solar Photo-Fenton Tertiary Treatment of Urban Wastewater. Catalysts 2022, 12, 552. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12050552
Deemter D, Coelho FEB, Oller I, Malato S, Amat AM. Assessment of a Novel Photocatalytic TiO2-Zirconia Ultrafiltration Membrane and Combination with Solar Photo-Fenton Tertiary Treatment of Urban Wastewater. Catalysts. 2022; 12(5):552. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12050552
Chicago/Turabian StyleDeemter, Dennis, Fabricio Eduardo Bortot Coelho, Isabel Oller, Sixto Malato, and Ana M. Amat. 2022. "Assessment of a Novel Photocatalytic TiO2-Zirconia Ultrafiltration Membrane and Combination with Solar Photo-Fenton Tertiary Treatment of Urban Wastewater" Catalysts 12, no. 5: 552. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12050552